Session Information
Date: Saturday, November 16, 2024
Title: RA – Treatment Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: In pts with RA, high RF levels are associated with poor prognosis, higher disease activity, and decreased response to monoclonal antibodies targeting tumor necrosis factor (TNF).1,2,3 Such pts may have better clinical responses to TNF inhibitors (TNFis) without a fragment crystallizable (Fc) portion, such as certolizumab pegol (CZP).4,5,6 Pts with high RF levels and previous TNFi inadequate response (TNFi-IR) may have poorer responses to subsequent therapies.7 We report the first analysis of the impact of RF levels and previous therapies on CZP efficacy in pts with RA.
Methods: REALISTIC (NCT00717236) was double-blind and placebo-controlled to Week (Wk)12.7 Pts with inadequately controlled RA were randomized 4:1 to CZP (400 mg subcutaneous [sc] at Wks 0/2/4, followed by 200 mg sc every 2 wks) or placebo (PBO) for 12 wks, followed by open-label CZP. We report: Disease Activity Score 28 C-reactive protein (DAS28-CRP) score, DAS28-CRP < 2.6, Clinical Disease Activity Index (CDAI) score, and CDAI remission (CDAI ≤2.8) to Wk36. Results (observed case) are stratified by baseline RF level (< 4th quarter [≤Q3] vs 4th quarter [Q4]) and prior TNFi use.
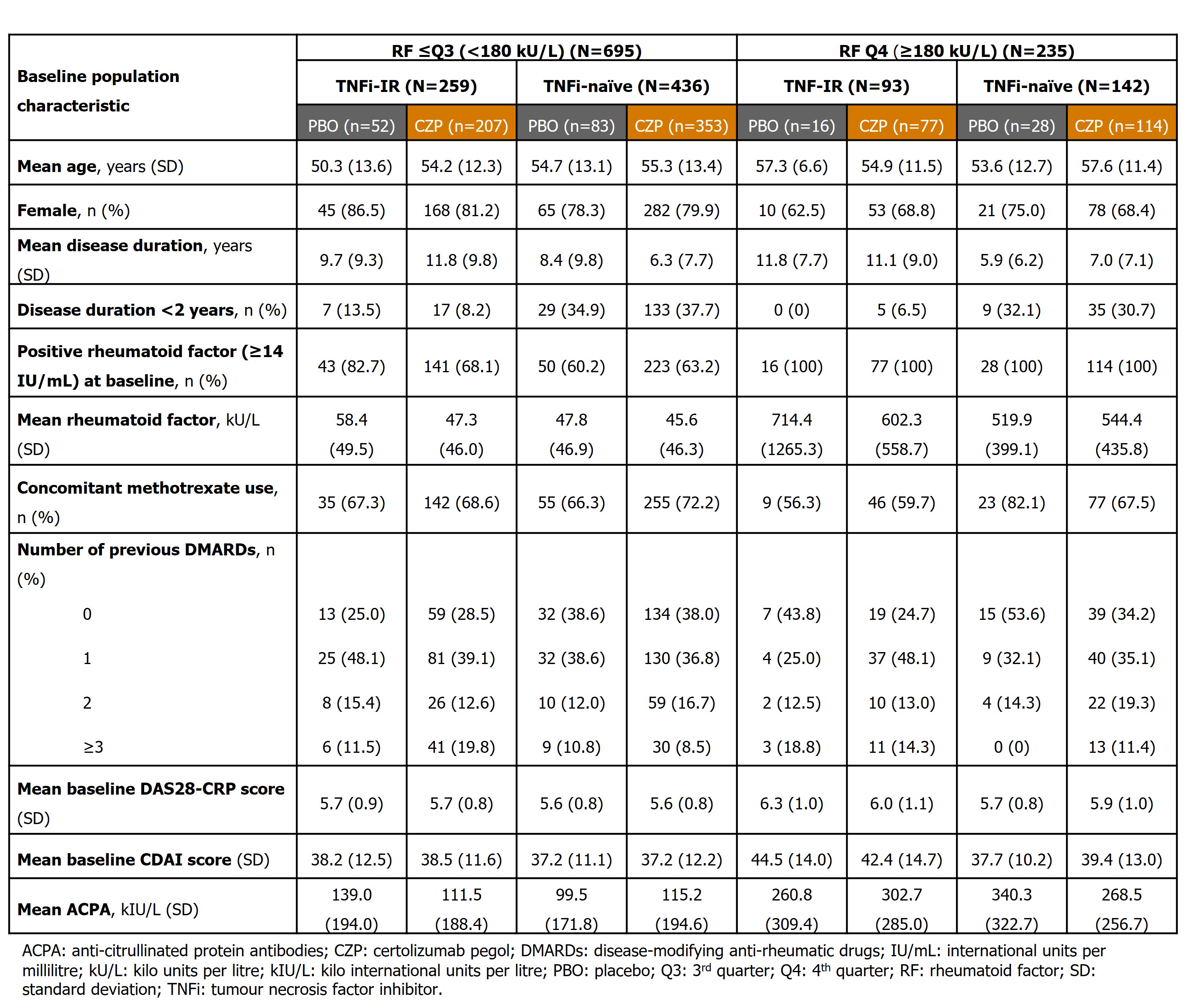
Results: 751 CZP-randomized pts (RF ≤Q3 [< 180 kU/L]: n=560, RF Q4 [≥180 kU/L]: n=191) and 179 PBO-randomized pts (RF ≤Q3: n=135, RF Q4: n=44) were included. Baseline demographics were similar between pts with RF ≤Q3 and Q4 (Table 1).
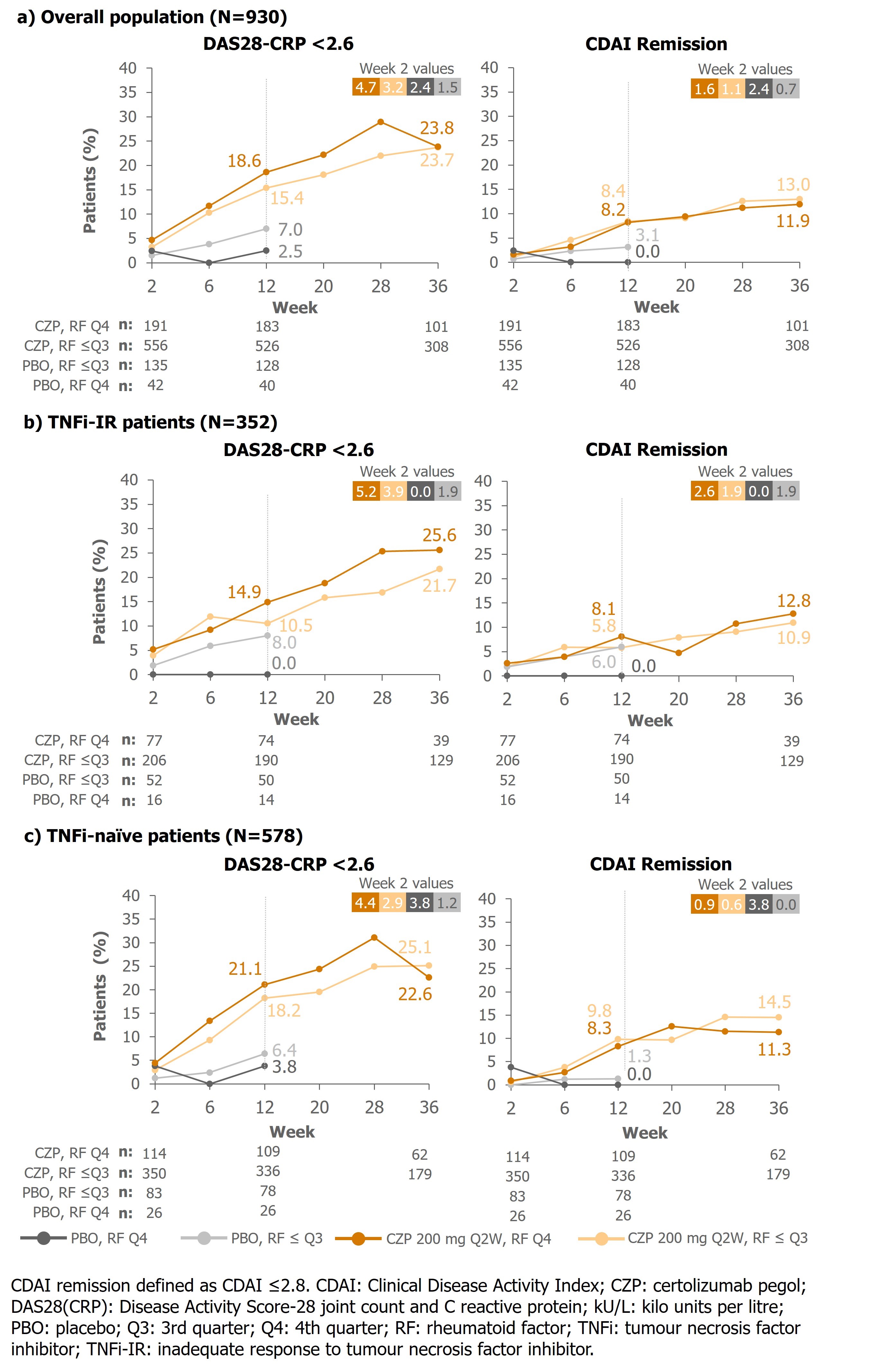
At Wk12, TNFi-naïve CZP-randomized pts had lower DAS28-CRP scores than PBO-randomized pts regardless of RF levels (CZP, mean [SD]: RF ≤Q3: 3.9 [1.3], RF Q4: 3.8 [1.3], PBO: RF ≤Q3: 4.9 [1.4], RF Q4: 4.9 [1.2]). In TNFi-IR pts, larger differences between DAS28-CRP scores were seen in pts with RF Q4 than RF ≤Q3 for PBO-randomized pts (RF ≤Q3 4.7 [1.4], RF Q4, 5.7 [1.4]) than CZP (RF ≤Q3: 4.2 [1.4], RF Q4: 4.2 [1.5]). In all CZP groups, responses increased over time; At Wk36, proportions of pts with DAS28-CRP< 2.6 were similar across RF levels and prior TNFi use (Figure 1).
At Wk12, TNFi-naïve CZP-randomized pts had lower CDAI scores than PBO-randomized pts, regardless of RF levels (RF ≤Q3: 18.6 [13.9] CZP vs 27.3 [15.5] PBO; RF Q4: 16.7 [12.1] CZP vs 26.9 [12.9] PBO). In TNFi-IR pts, larger differences between CDAI scores for CZP vs PBO were seen in pts with high RF (RF ≤Q3, mean [SD]: 21.6 [15.4] CZP vs 26.0 [15.8] PBO; RF Q4, 20.4 [14.9] CZP vs 35.4 [18.2] PBO). In CZP-randomized pts, responses increased over time; At Wk36, proportions of pts who achieved CDAI remission were similar across RF levels and prior TNFi use (Figure 1).
Conclusion: In PBO-randomized TNF-IR pts, clinical responses were lower in pts with high RF than low RF. In contrast, pts treated with CZP had similar responses irrespective of RF level, suggesting RF does not influence response to CZP. These data may have treatment choice implications for pts with RA and high RF levels who had inadequate responses to previous TNFis.
References: 1. Vastesaeger N. et al, Rheum 2009;48:1114–21. 2. Cuchacovich M. et al, Clin Rheumatol 2014;33(12):1707–14. 3. Takeuchi T. et al, Arthritis Res 2017;19:194. 4. Nakayama Y. Rheumatol Int 2022;42:1227–34. 5. Smolen J. Arthritis Rheumatol 2023;75(suppl 9). 6. Bidgood S. et al, Ann Rheum Dis 2024;83 (suppl 1):727. 7. Pappas D.A. et al, Rheumatol Int 2021;41:585–93.
To cite this abstract in AMA style:
Smolen J, Mikuls T, Galloway J, Müller-Ladner U, Curtis J, Hashimoto M, Takeuchi T, Choy E, Tanaka Y, Cara C, Lauwerys B, Tilt N, Ufuktepe B, Taylor P. Do High Rheumatoid Factor Levels Impact Response to Certolizumab Pegol in Patients with Inadequately Controlled Rheumatoid Arthritis? A Post Hoc Analysis of a Phase 3b Trial [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/do-high-rheumatoid-factor-levels-impact-response-to-certolizumab-pegol-in-patients-with-inadequately-controlled-rheumatoid-arthritis-a-post-hoc-analysis-of-a-phase-3b-trial/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/do-high-rheumatoid-factor-levels-impact-response-to-certolizumab-pegol-in-patients-with-inadequately-controlled-rheumatoid-arthritis-a-post-hoc-analysis-of-a-phase-3b-trial/