Session Information
Date: Monday, November 11, 2019
Title: Systemic Sclerosis & Related Disorders – Basic Science Poster
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Iloprost improves Raynaud‘s phenomenon and digital ulcers in scleroderma (SSc) patients. This is hypothesized to reflect anti-platelet and vasodilatory effects. Different trials and cohorts have reported efficacy of Iloprost for 4-8 weeks [1] despite a short half-life (0.57hr; [2]). Adherens junctions, which are formed by clustering of VE-cadherin on neighboring endothelial cells (ECs), regulate numerous endothelial properties, including cell morphology, signaling and phenotype [3]. When junctions are disrupted, VE-cadherin and β-catenin are disengaged from the cell membrane and can contribute to vascular dysfunction and endothelial to mesenchymal transition (Endo-MT). We hypothesized that vascular dysfunction in SSc reflects disruption of EC adherens junctions and that the vascular protective effect of Iloprost is mediated by strengthening of these junctions in SSc ECs.
Methods: Dermal ECs were isolated from biopsies of healthy subjects and SSc patients. ECs were treated with Iloprost (150nM) for various times. Visualization of cellular localization of VE-cadherin, β-catenin, and F-actin was done by immunofluorescence. Tubulogenesis was examined using Matrigel in vitro tube formation assay. Inhibition of VE-cadherin clustering was achieved by a function blocking antibody. Cell proliferation was assessed by BrdU incorporation. Cell migration was examined using Incucyte imaging. Endo-MT was induced by treating healthy ECs with TGFβ for 72 hrs. Gene expression was determined by qPCR. Plasma VE-cadherin was determined using ELISA. T-tests were used to compare differences between groups, and P< 0.05 considered significant.
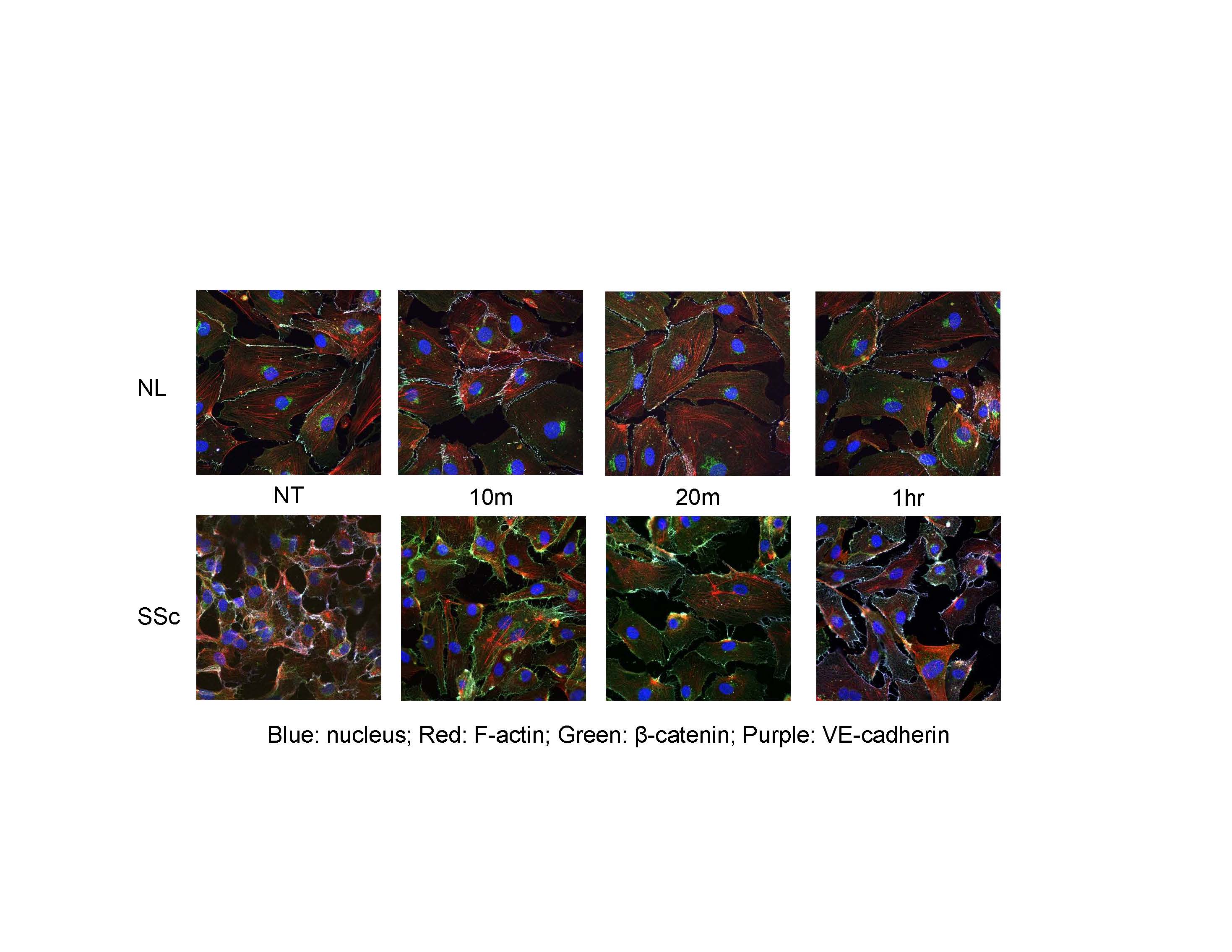
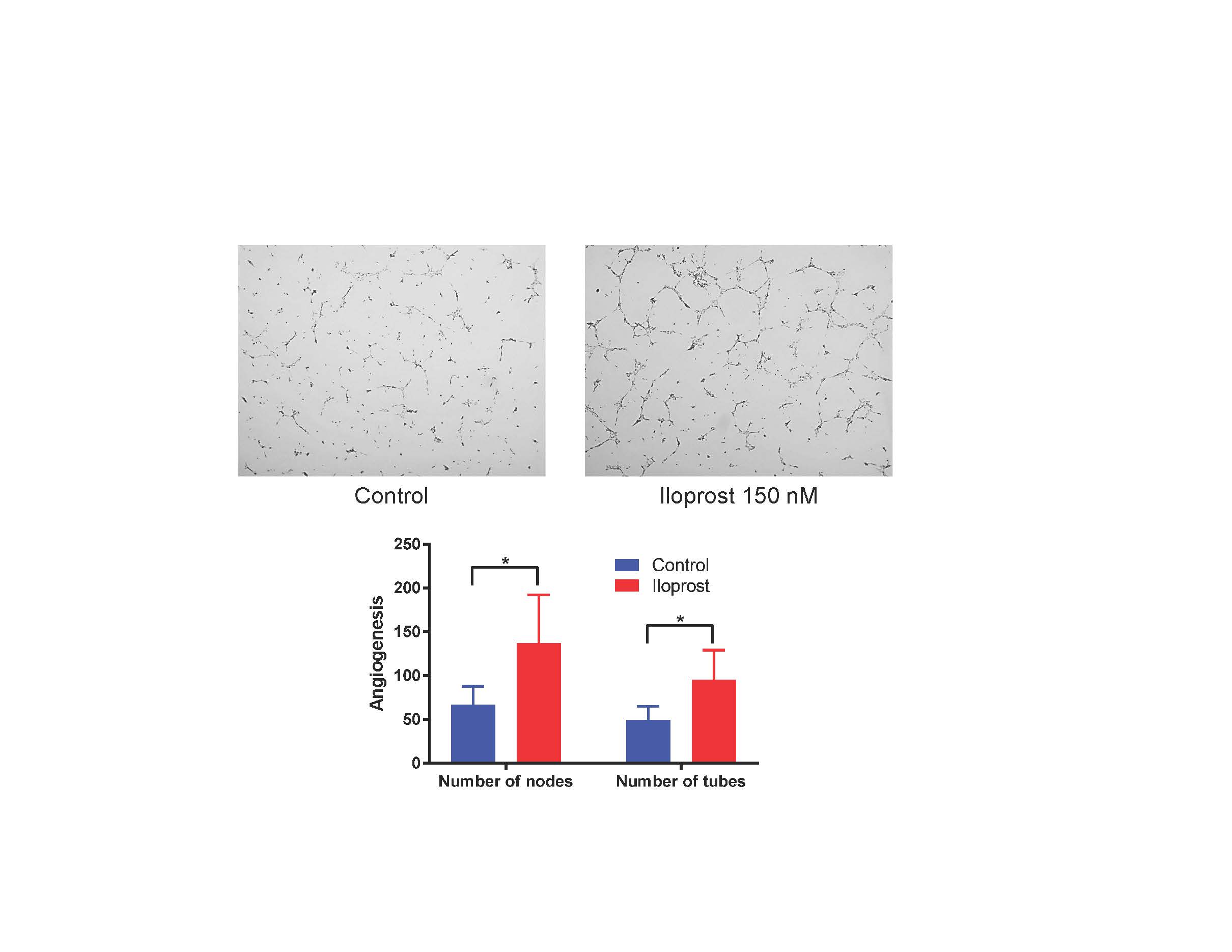
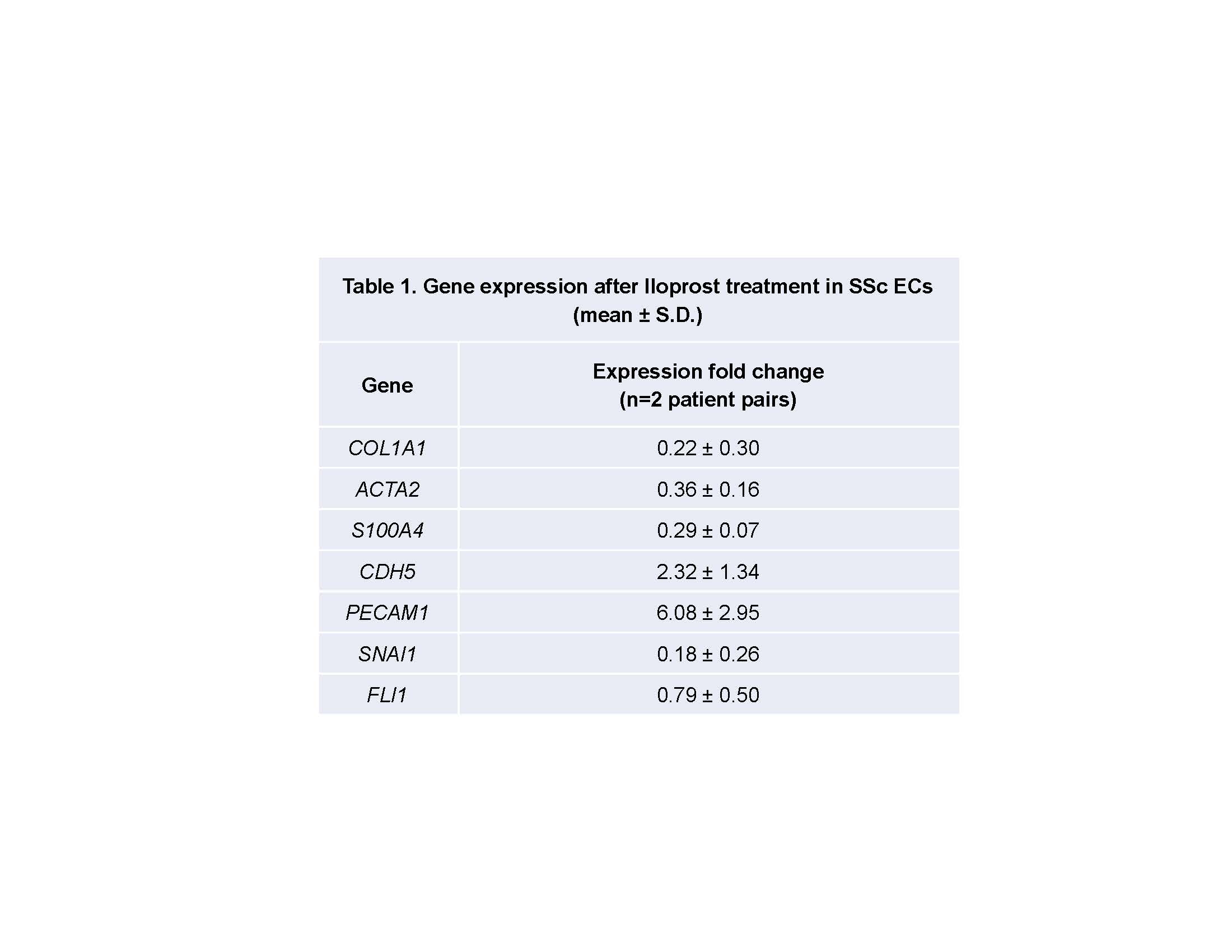
Results: Cell staining demonstrated disruption of adherens junctions and disorganized F-actin filaments in SSc compared to normal ECs. When stimulated with Iloprost, there was a significant increase in VE-cadherin and β-catenin clustering at cell junctions up to 1 hour of incubation in both normal and SSc ECs. However, F-actin organization in SSc ECs was still distorted (Fig 1). Iloprost treatment increased EC tubulogenesis in both normal and SSc ECs (Fig 2). This effect was inhibited by a neutralizing antibody to VE-cadherin. Iloprost had minimal effect on EC proliferation. In normal ECs, TGFβ induced Endo-MT with upregulation of ACTA2, S100A4, and SNAI1, and downregulation of PECAM1, CDH5, and FLI1. This process was blocked by Iloprost co-incubation; Iloprost normalized the expression of all genes except for FLI1. Similar results were observed in SSc ECs treated with Iloprost for 72 hrs (Table 1). Plasma level of VE-cadherin was significantly elevated in SSc compared to healthy controls.
Conclusion: Our data suggests that long lasting beneficial effects of Iloprost may stem from its ability to stabilize endothelial adherens junctions, increasing EC tubulogenesis, and inhibiting Endo-MT. These results provide a mechanistic basis that supports the use of Iloprost in treating SSc patients with Raynaud’s phenomenon and digital ulcers.
- Wigley, F.M., et al., Ann Intern Med, 1994. 120(3): p. 199-206.
- Hildebrand, M., Eur J Clin Pharmacol, 1997. 53(1): p. 51-6.
- Flavahan, N.A., J Cardiovasc Pharmacol, 2017. 69(5): p. 248-263.
To cite this abstract in AMA style:
Tsou P, Flavahan N, Khanna D. Dissecting the Cellular Mechanism of Prostacyclin Analog Iloprost in Reversing Vascular Dysfunction in Scleroderma [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/dissecting-the-cellular-mechanism-of-prostacyclin-analog-iloprost-in-reversing-vascular-dysfunction-in-scleroderma/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/dissecting-the-cellular-mechanism-of-prostacyclin-analog-iloprost-in-reversing-vascular-dysfunction-in-scleroderma/