Session Information
Date: Monday, November 14, 2022
Title: Abstracts: RA – Treatment III: Comorbidities and Consequences
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Paraoxonase-1 (PON1) is a high-density lipoprotein (HDL)-associated enzyme with paraoxonase, lactonase, and arylesterase activities (1). PON1 is integral to the anti-inflammatory, anti-atherogenic functions of HDL. Higher PON1 activity has been associated with improved HDL function, reduced cardiovascular (CV) risk (2) and improvement in arthritis activity in the K/BxN mouse model of rheumatoid arthritis (RA) (3). The current work evaluates PON1 activity at baseline and after 6 months of treatment with multiple biologic therapies in the Comparative Effectiveness Registry to Study Therapies for Arthritis and Inflammatory conditions (CERTAIN).
Methods: Adult RA patients with moderate or high disease activity (CDAI >10) who initiated a biologic agent that had not been previously used for the treatment were enrolled in the CERTAIN study. Serum specimens from patients at baseline and 6 months after the biologic use were analyzed for PON1 activity by paraoxonase, lactonase, and arylesterase assays as described previously (3).
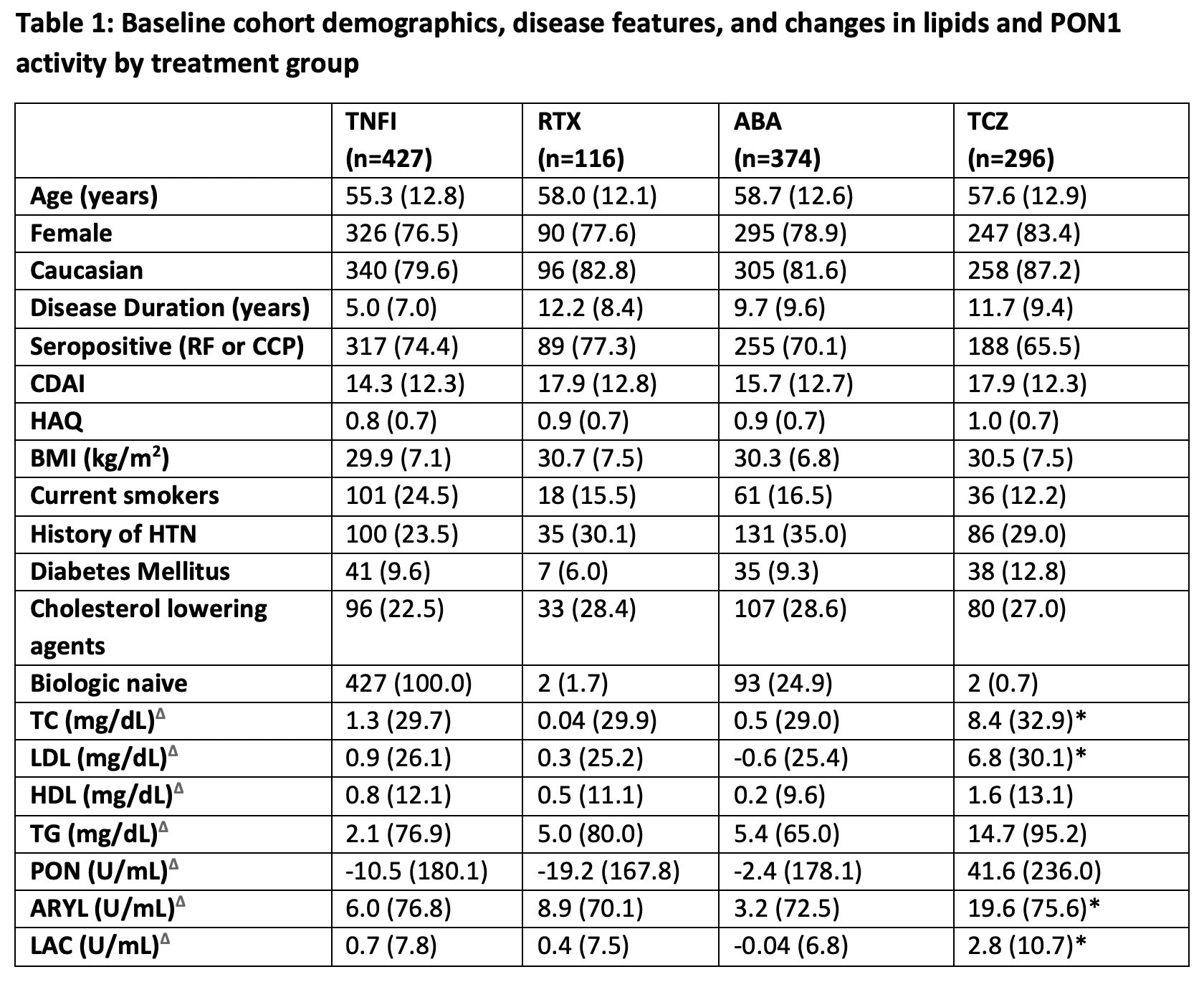
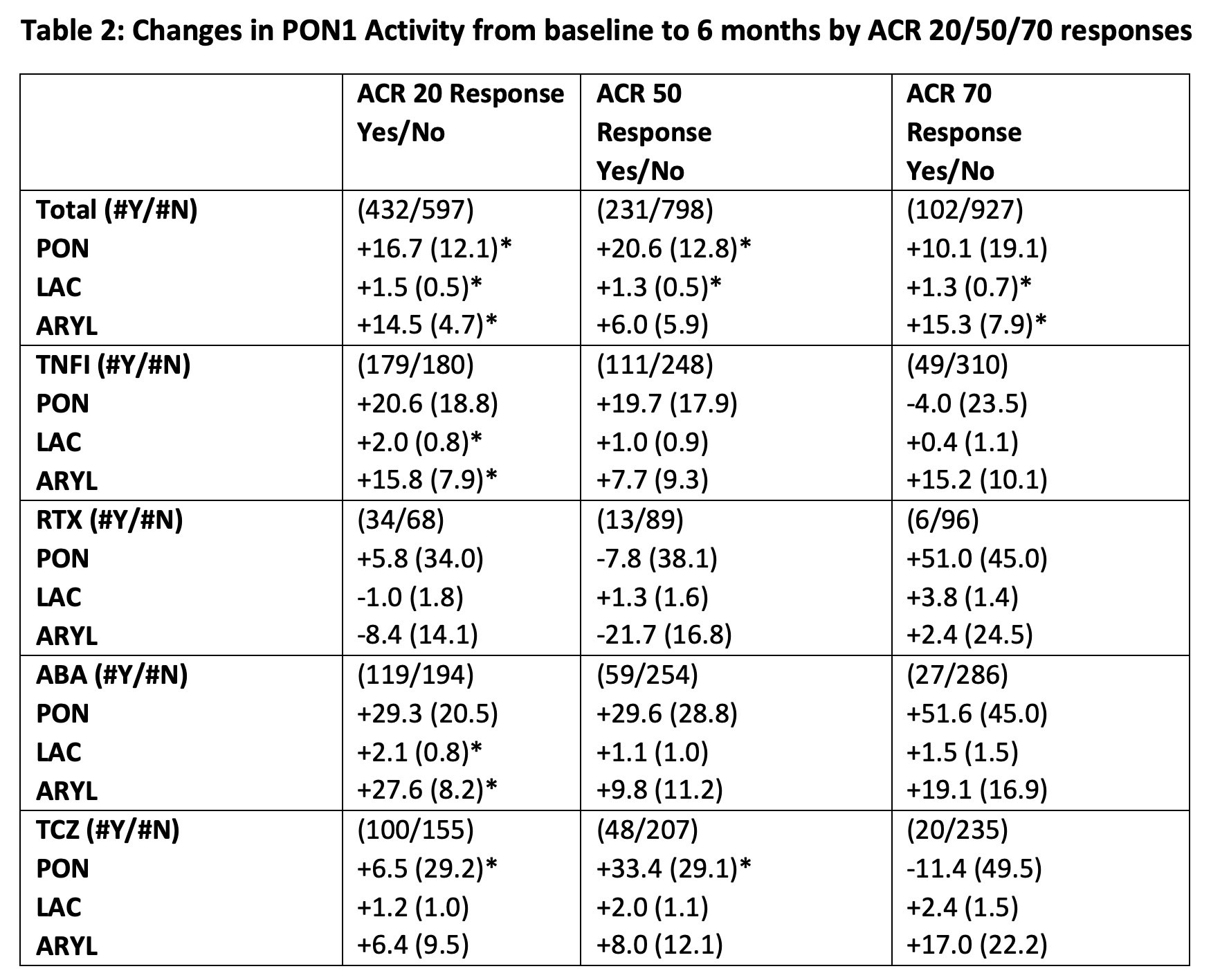
Results: 1213 patients in the CERTAIN registry had serum specimens at 0 and 6 months available for analysis including tocilizumab (TCZ, all biologic experienced), n=296; abatacept (ABA, naïve and biologic experienced), n= 374; TNFα inhibitors (TNFI) (all biologic naïve), n= 427; rituximab (RTX, all biologic experienced), n=116. Baseline demographic factors, CV risk, and RA disease activity measures were similar across treatment groups at baseline (Table 1). Small increases in PON1 activity by arylesterase and lactonase assays after 6 months of treatment were noted in analysis of the entire cohort (9.3 (3.0) and 0.98 (0.37) mean difference (SE) U/ml respectively, p values < 0.05 for 0 to 6 months comparison). The differences were greatest in the tocilizumab group, which also had the greatest increases in lipid levels (Table 1). Patients with ACR 20, 50, and 70 disease responses to treatment at 6 months had greater improvements in PON1 activities than ACR non-responders across the entire cohort, with similar trends across all treatment groups (Table 2). Decreases in disease activity measured by a disease activity score with 28 joint count and CRP (DAS28CRP) correlated significantly with increases in all 3 activities of PON1 (PON r=-0.12, arylesterase r=-0.13, lactonase r=-0.12, all p< 0.0001).
Conclusion: Improvement in disease activity across 4 classes of biologic therapies with different mechanisms of action associates with improvement in the activity of PON1, a protein associated with improved HDL function and CV risk reduction. These findings suggest a potential mechanism by which improvement in RA disease activity by multiple therapies reduces CV risk through improvement in the activity of PON1.
References:
1) Mackness M, Mackness B. Gene 2015; 567(1):12-21
2) Charles-Schoeman C, et al. Arthritis Rheum 2013; 65(11):2765-72
3) Charles-Schoeman C, et al. Sci Rep 2020; 10(1):16848
Δ =Difference from baseline to 6 months
*=p<0.05 for comparison of baseline and 6-month values
HTN= hypertension, RF= rheumatoid factor, CCP= anti-cyclic citrullinated peptide antibody, TC= total cholesterol, LDL= low density lipoprotein, HDL= high density lipoprotein, TG= triglycerides, PON= paraoxonase activity, ARYL=arylesterase activity, LAC= lactonase activity
Total= Entire registry, TNFI = TNF Inhibitor group, RTX= Rituxan, ABA= Abatacept, TCZ= tocilizumab.
#Y = number of ACR responders (yes) versus #N = number of ACR non-responders (no).
To cite this abstract in AMA style:
Razmjou A, Wang J, Shahbazian A, Curtis J, Pappas D, Kremer J, Charles-Schoeman C. Disease Response in Rheumatoid Arthritis Across 4 Biologic Therapies Associates with Improvement in Paraoxonase-1 Activity [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/disease-response-in-rheumatoid-arthritis-across-4-biologic-therapies-associates-with-improvement-in-paraoxonase-1-activity/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/disease-response-in-rheumatoid-arthritis-across-4-biologic-therapies-associates-with-improvement-in-paraoxonase-1-activity/