Session Information
Date: Monday, October 27, 2025
Title: (1612–1632) Vasculitis – Non-ANCA-Associated & Related Disorders Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Takayasu’s arteritis (TAK) is a rare, systemic, chronic large vessel vasculitis of unknown etiology primarily affecting the aorta and its branches. Arterial damage, with stenosis or occlusion of the large arteries, may occur as a result of vascular inflammation. In response, collateral circulation is known to develop in some patients and helps overcome vascular damage by providing alternative blood flow to areas where major vessels are blocked or narrowed. Therapeutic targeting of tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) can lead to clinical improvement in subsets of patients with TAK. However, it remains unclear which components are more likely to be effective targets for the treatment of TAK and whether they may impact collateral vessel formation. The aim of this study is to investigate whether and how inflammation affects angiogenic outcomes in the context of TAK.
Methods: To understand the molecular players involved in TAK disease activity and blood vessel formation, we utilized flow cytometry to analyze the frequency of peripheral blood-derived circulating endothelial progenitor (EPCs) and CD31+ terminally differentiated endothelial cells (CECs), Luminex assays to measure plasma-derived cytokines and growth factors, and live cell imaging of Matrigel-based tube formation assays to test the angiogenic potential of CECs and endothelial cell lines in vitro (Figure 1). As TNF-α and IL-6 have an important role in driving TAK-related inflammation, we investigated their effect on angiogenesis.
Results: Neither the frequency of EPCs nor CECs among TAK patients was significantly correlated with disease activity in our cohort TAK patients (n = 9). Additionally, there were no striking differences in the overall profile of plasma-derived cytokines and growth factors in these patients (n= 64). However, CECs isolated from TAK patients in remission exhibited a robust angiogenic potential that was similar to that of endothelial cell lines, while those from TAK patients with active disease showed diminished capacity to form tubular structures. Finally, TNF-α but not IL-6 or the IL6/sIL6R complex showed an apparent anti-angiogenic effect in vitro.
Conclusion: We established an efficient in vitro assay capable of confirming that chronic inflammation in TAK can lead to endothelial dysfunction, since the disease activity state influences the angiogenic capabilities of patient-derived CECs. In addition, we identified TNF-α as a dominant cytokine that contributes to vascular pathology. Overall, our experimental framework provides useful insights into the basic mechanisms of TAK pathogenesis. Future research integrating clinical response with effective therapy to reduce inflammation and promote collateral vessel formation may be potentially beneficial for patients with TAK.
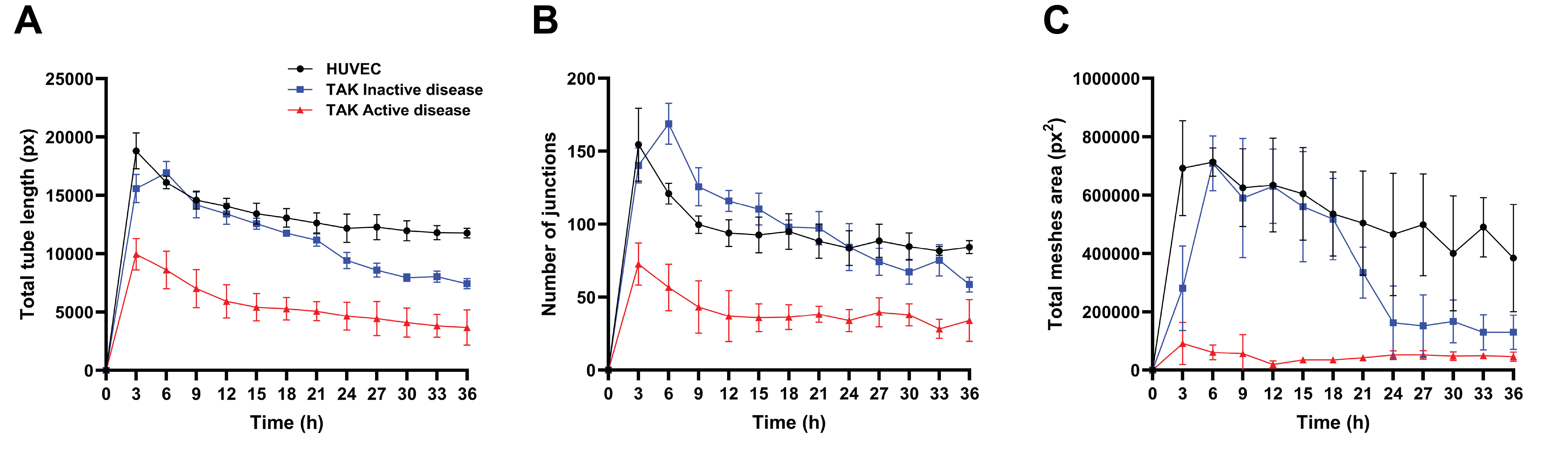 Figure 1: Matrigel tube formation assay in an Incucyte® Live-Cell Analysis System. TAK-derived CECs from active and inactive disease states, as well as HUVECs, were cultured in endothelial growth media (EGM-2) for 36 h in 96-well plates coated with a semi-solid Matrigel. The total tube lengths (A), number of junctions (B), and total meshes area (C) formed in the assay were measured. Data are presented as the mean ± standard error of the mean.
Figure 1: Matrigel tube formation assay in an Incucyte® Live-Cell Analysis System. TAK-derived CECs from active and inactive disease states, as well as HUVECs, were cultured in endothelial growth media (EGM-2) for 36 h in 96-well plates coated with a semi-solid Matrigel. The total tube lengths (A), number of junctions (B), and total meshes area (C) formed in the assay were measured. Data are presented as the mean ± standard error of the mean.
To cite this abstract in AMA style:
Yamamoto de Almeida L, Quinn K, Simone J, Dai L, Turturice B, Kaundal U, Palmer C, Stonick K, Randazzo d, Carmona-Rivera C, Grayson P. Disease activity status in Takayasu’s arteritis influences the angiogenic potential of patient-derived endothelial cells [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/disease-activity-status-in-takayasus-arteritis-influences-the-angiogenic-potential-of-patient-derived-endothelial-cells/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/disease-activity-status-in-takayasus-arteritis-influences-the-angiogenic-potential-of-patient-derived-endothelial-cells/
