Session Information
Date: Sunday, November 13, 2022
Title: Spondyloarthritis Including PsA – Diagnosis, Manifestations, and Outcomes Poster II
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Improvement in functioning and health as assessed by the ASAS Health Index (HI) is an important outcome of interventions in patients with axial spondyloarthritis (axSpA). ASAS HI thresholds for measuring improvement have been proposed but not yet tested in an independent intervention trial to study its discriminant capacity. The objective was to test the discriminant capacity of the ASAS HI using data from a randomized, active-controlled trial.
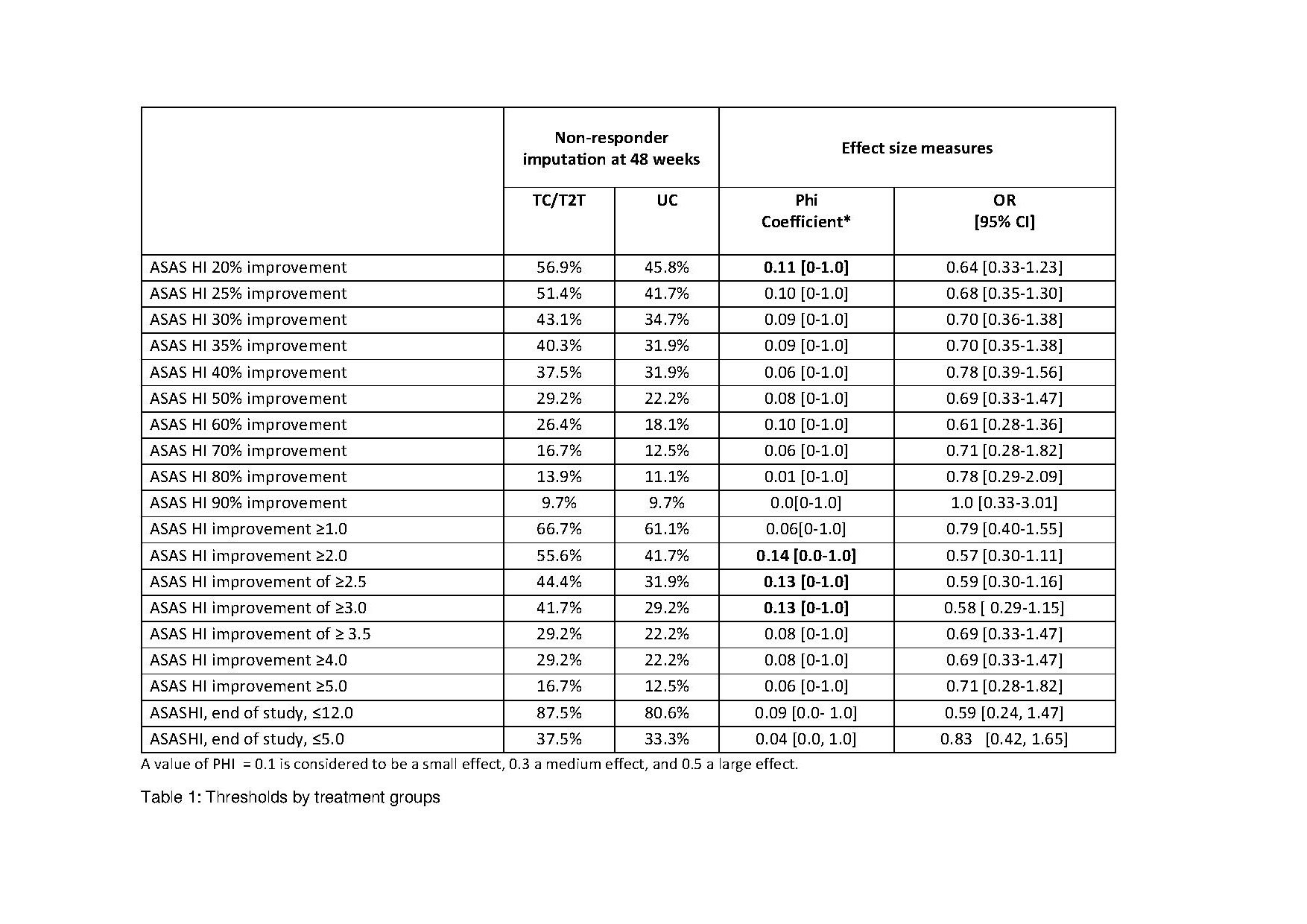
Methods: In this post-hoc analysis from the tight-controlled, treat-to-target (T2T) trial TICOSPA, data of active axSpA patients randomized to either the T2T arm (visits every 4 weeks, prespecified strategy of treatment intensification until achieving low disease activity) or standard of care (SOC; visits every 12 weeks, treatment at the rheumatologist’s discretion) were compared to test whether different thresholds for improvement or achieved state of ASAS HI could discriminate between treatment arms. Week 48 effect sizes (ES) of improvement from baseline were calculated for each treatment arm as Phi Coefficient (higher means better discrimination) and OR (95% CI).
Results: The table shows the ES between treatment arms for all tested improvements and health states achieved in ASAS HI. Overall, absolute improvement outcomes performed better than percentage changes outcome followed by status outcomes. The absolute improvement of ≥2.0, ≥2.5, and ≥3.0 performed best followed by the 20% improvement. As the ASAS HI ≥3.0 is the smallest detectable change for this outcome, this seem to be the most appropriate proposed outcome.
Conclusion: In this active-controlled trial an absolute improvement in the ASAS HI discriminated best between treatment arms.. A similar evaluation is needed in a placebo-controlled trial to be able to propose the best outcome for the ASAS HI in a trial.
To cite this abstract in AMA style:
Kiltz U, Molto A, Lopez-Medina C, Dougados M, van der Heijde D, Boonen A, Van den bosch F, Braun J. Discriminatory Capacity of the ASAS Health Index in Patients with Axial Spondyloarthritis Treated in a Tight Control Setting versus Standard of Care [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/discriminatory-capacity-of-the-asas-health-index-in-patients-with-axial-spondyloarthritis-treated-in-a-tight-control-setting-versus-standard-of-care/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/discriminatory-capacity-of-the-asas-health-index-in-patients-with-axial-spondyloarthritis-treated-in-a-tight-control-setting-versus-standard-of-care/