Session Information
Date: Wednesday, October 29, 2025
Title: Abstracts: Systemic Sclerosis & Related Disorders – Clinical III (2651–2656)
Session Type: Abstract Session
Session Time: 10:15AM-10:30AM
Background/Purpose: To determine the frequency and extent of discordance between patient and physician global assessments of disease in early systemic sclerosis and identify factors associated with discordance.
Methods: Adult patients with early systemic sclerosis from the COllaborative National QUality and Efficacy Registry (CONQUER) were included. Global disease assessments by patients and physicians (0-10, higher is worse, 7-day recall), clinical evaluations, and patient-reported outcomes were collected at enrollment and every 6 months. Score concordance was defined as patient and physician global scores within 1 point, and discordance as a difference of ≥ 2 points, creating 3 categories: concordance, patient scores worse, and physician scores worse. The frequency of discordance and correlation between global assessments were determined for the enrollment visit, and factors associated with discordance were assessed using multivariable mixed-effects proportional odds regression with concordance as the reference category.
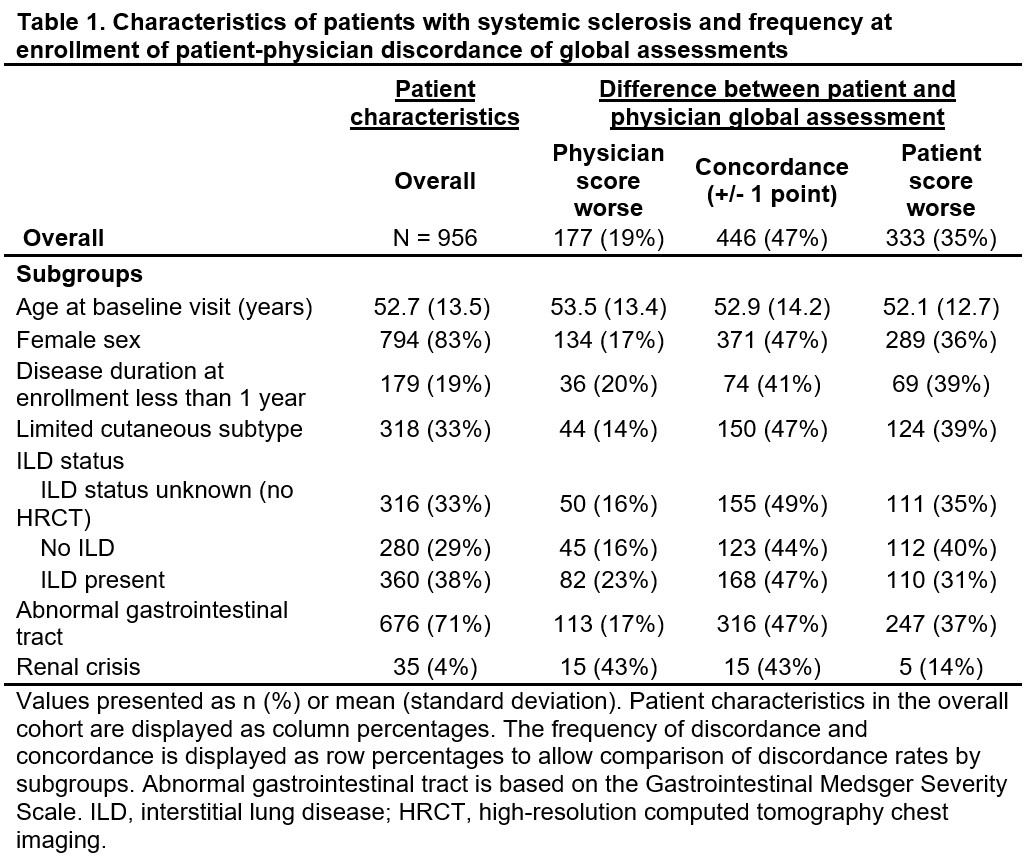
Results: 956 patients (83% female, 33% limited cutaneous disease, 19% with disease duration < 1 year) with both global assessments available were included (Table 1). The correlation between patient and physician global assessments was weak (Pearson’s r=0.28) (Figure 1). Discordance between global assessments was present in 510 (53%) of patients (35% patient score worse; 19% physician score worse)(Figure 2). In the multivariable model, compared to score concordance, physicians tended to report global scores worse than patient scores when there was a higher modified Rodnan skin score (mRSS)(odds ratio (OR), [95% confidence interval (CI)]: 1.03 [1.01, 1.05]), worse New York Heart Association (NYHA) functional Class (Class II OR 1.78 [1.20, 2.65]; Class III/IV: 2.83 [1.07, 7.46]), and higher PROMIS Pain Interference (OR 1.03, [1.00, 1.05]), while higher diffusing capacity of the lung for carbon monoxide (DLCO) %-predicted (OR 0.94 [0.90, 0.98]) was associated with lower likelihood of discordance with physician scores worse. Patients tended to report global scores worse than physician scores when there was higher overall pain/discomfort (OR 1.29 [1.18, 1.42]), while decreased likelihood of discordance with the patient scores worse was observed for limited vs. diffuse cutaneous subtype (OR 0.45 [0.28, 0.71]), higher mRSS (OR 0.97 [0.95, 0.99]), and worse NYHA functional Class (Class III/IV OR 0.29 [0.13, 0.66]).
Conclusion: Discordance between global assessments is present in over half of cases in systemic sclerosis, with patients tending to rate their condition worse compared to their physician’s rating. Discordance in which physician scores are worse is associated with disease characteristics and measures of severity, while discordance in which patient scores are worse is associated with increased pain and lower likelihood of severe manifestations. Physicians may underestimate disease impact when disease manifestations are less severe. These results highlight differing perceptions of disease severity and emphasize the need for comprehensive approaches to symptom management and measurement of disease burden in this complex disease.
.jpg) Figure 1. Scatterplot and distributions of patient and physician global assessments of overall disease activity in systemic sclerosis at enrollment visit.
Figure 1. Scatterplot and distributions of patient and physician global assessments of overall disease activity in systemic sclerosis at enrollment visit.
The size of the blue circles represents the number of patients with each score combination. Points above the gray lines indicate discordance with the patient score worse, while points below the gray lines indicate discordance with the physician score worse. Points between the gray lines are concordant. Mean (standard deviation) global assessments at enrollment were 4.2 (2.6) and 3.5 (2.0) among patients and physicians, respectively. The distributions demonstrate that patients are more likely to utilize the full range of scores compared to physicians.
.jpg) Figure 2. Distribution of differences between patient and physician global assessment scores among patients with systemic sclerosis at enrollment visit.
Figure 2. Distribution of differences between patient and physician global assessment scores among patients with systemic sclerosis at enrollment visit.
Global assessment score concordance (+/- 1 point) was present in 47% of patient-physician assessments at the enrollment visit (blue). Discordance (≥ 2-point difference) was present in 53%. Patient global assessment scores were worse than the physician scores in 35% (green), while physician global assessment scores were worse than the patient scores in 19% (purple). Patient scores were more likely to show high discordance, with 15% of scores 4 or more points higher than the corresponding physician scores.
To cite this abstract in AMA style:
Romich E, Ogdie A, Stephens Shields A, Merkel P, Alvey J, Assassi S, Bernstein E, Bracken S, Castelino F, Chung L, Evnin L, Frech T, Gordon J, Hant F, Harding M, Hummers L, Khanna D, Lakin K, Lebiedz-Odrobina D, Luo Y, Makol A, Mayes M, McMahan Z, Molitor J, Moore D, Richardson C, Shah A, Shah A, Skaug B, Steen V, VanBuren J, Volkmann E, Zahn C, Sandorfi N. Discordance Between Patient and Physician Global Assessments in Early Systemic Sclerosis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/discordance-between-patient-and-physician-global-assessments-in-early-systemic-sclerosis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/discordance-between-patient-and-physician-global-assessments-in-early-systemic-sclerosis/