Session Information
Date: Sunday, November 7, 2021
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: Tofacitinib (TOFA) is an oral, small molecule drug used for rheumatoid arthritis (RA) treatment and is prescribed alone or with methotrexate (MTX). We previously reported the similarity in retention between TOFA monotherapy and TOFA with MTX using data from two different registries separately; the Ontario Best Practices Research Initiative (OBRI) and the Quebec registry RHUMADATA.
Methods: RA patients enrolled in the OBRI and RHUMADATA initiating their TOFA between 1st June 2014 (TOFA approval date in Canada) and 31st Dec 2019 were included. Concurrent MTX use was defined as MTX use for more than 75% of the time while using TOFA. Multiple imputation (Imputation Chained Equation method, N=20) was used to deal with missing data for covariates at treatment initiation.
Time to discontinuation was assessed using Cox regression models. To deal with confounding by indication, we estimated propensity scores for selected covariates with an absolute standard difference greater than 0.1. We then adjusted Cox regression models for propensity quantile to compare the discontinuation of TOFA with MTX versus TOFA without MTX.
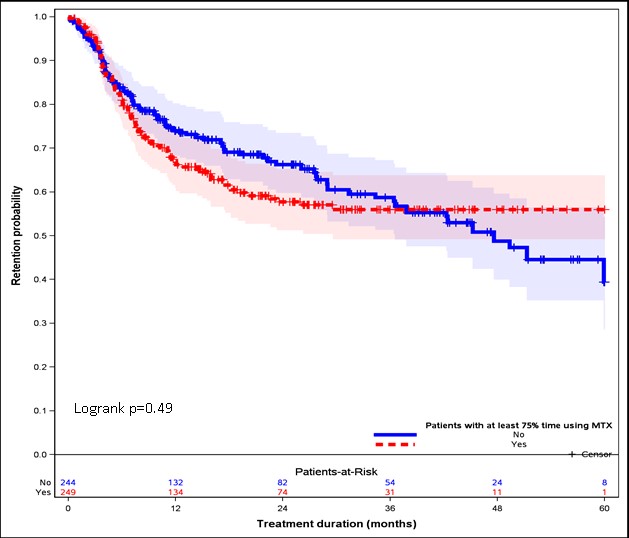
Results: A total of 493 patients were included. Of those, 244 (49.5%) and 249 (51.5%) were treated with MTX and without MTX, respectively. Compared to TOFA monotherapy, the TOFA with MTX group had a significantly lower mean HAQ-DI, fatigue score, and the number of prior biologic use at the time of TOFA initiation. A lower proportion of positive ACPA (59% vs. 66%), prevalence of hypertension (31% vs 37%), and concomitant use of Leflunomide (11% vs. 23%) were also observed for patients using TOFA with MTX.
Over a mean follow-up of 19.0 months, discontinuation was reported in 182 (36.9%) of all TOFA patients. After adjusting for propensity score quantile across 20 imputed datasets, there was no significant difference in discontinuation between treatment groups (adjusted HRs: 1.12, 95% CI: 0.83-1.51; p=0.49) (Figure 1).
Conclusion: In this pooled real-world data study, we found that in patients with RA, the retention of TOFA is similar if it is used as monotherapy or in combination with MTX.
To cite this abstract in AMA style:
Movahedi M, Choquette D, Coupal L, Cesta A, Li X, Keystone E, Bombardier C. Discontinuation Rate of Tofacitinib as Monotherapy Is Similar Compared to Combination Therapy with Methotrexate in Rheumatoid Arthritis Patients: Pooled Data from Two Rheumatoid Arthritis Registries in Canada [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/discontinuation-rate-of-tofacitinib-as-monotherapy-is-similar-compared-to-combination-therapy-with-methotrexate-in-rheumatoid-arthritis-patients-pooled-data-from-two-rheumatoid-arthritis-registries-i/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/discontinuation-rate-of-tofacitinib-as-monotherapy-is-similar-compared-to-combination-therapy-with-methotrexate-in-rheumatoid-arthritis-patients-pooled-data-from-two-rheumatoid-arthritis-registries-i/