Session Information
Date: Tuesday, November 14, 2023
Title: Abstracts: RA – Treatments III: Predictors of Response & Tapering
Session Type: Abstract Session
Session Time: 4:00PM-5:30PM
Background/Purpose: The identification of measures that help predict who is likely to benefit most from early escalation to biologic therapy would inform personalized care among patients with rheumatoid arthritis (RA) early in the course of the disease. While prior studies have assessed how body mass index (BMI) and adiposity might influence responses to therapy, these studies rarely have compared two active treatment strategies. We aimed to determine if BMI and circulating adipokines (peptide hormones produced by adipose tissue) can identify patients most likely to benefit from early initiation of tumor necrosis factor inhibitors (TNFi) as initial therapy compared to combination therapy with conventional DMARDs.
Methods: This is a secondary analysis of the Rheumatoid Arthritis Comparison of Active Treatments (RACAT) trial and the Treatment of Early Aggressive Rheumatoid Arthritis (TEAR) trial. Both trials evaluated strategies with conventional DMARDs (triple therapy) compared to strategies that included TNFi (etanercept), among patients with active RA. We compared response rates between TNFi and triple therapy among patients with different BMI (normal/underweight v. overweight/obese). Adipokines were also measured at enrollment in RACAT using validated assays and we explored the association between an adipokine score (number of adipokines above/below the median, grouping those that directionally reflect greater adiposity) and response within the two treatment arms. Analyses were adjusted for age, sex, BMI, and baseline disease activity (DAS28).
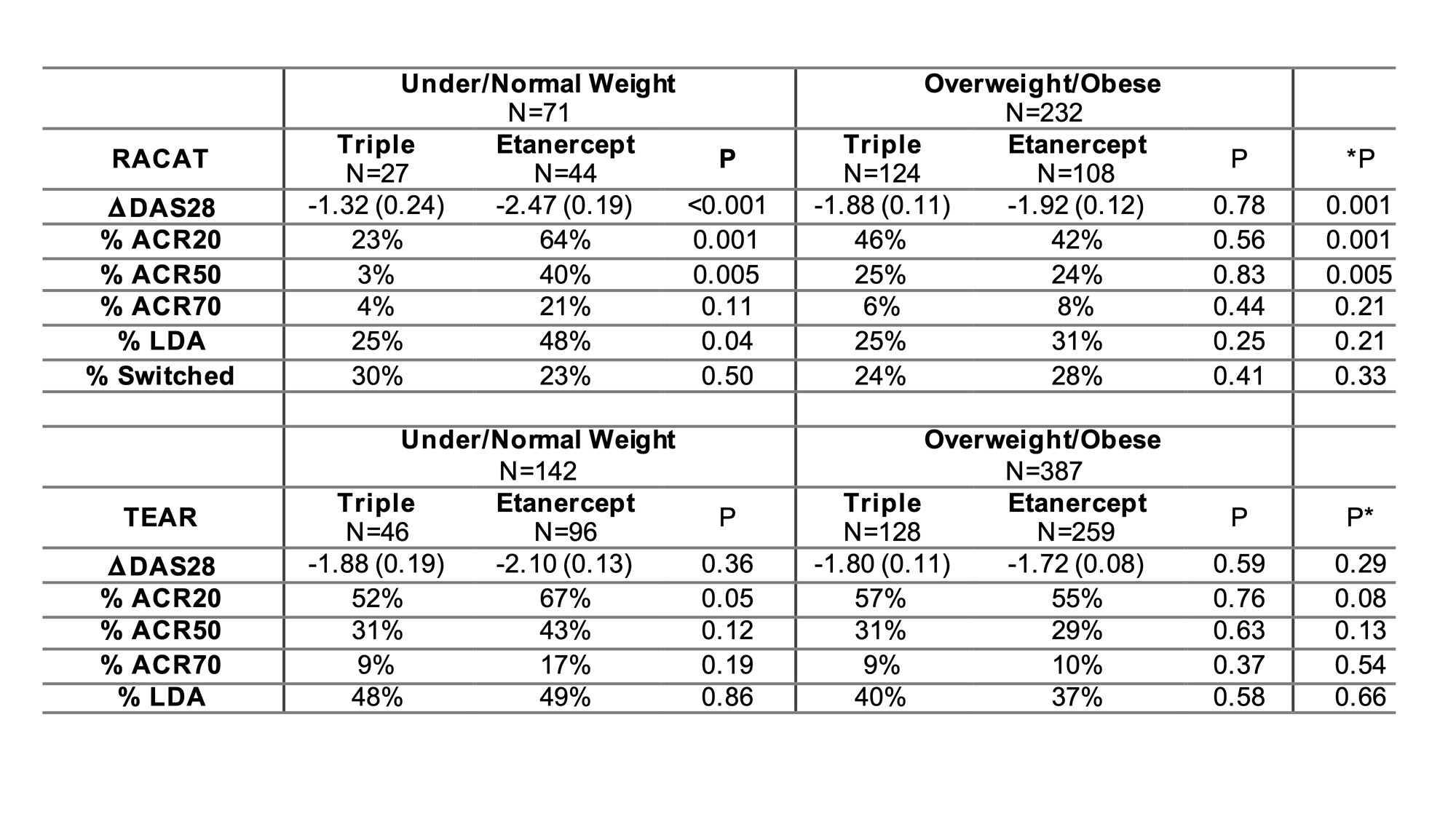
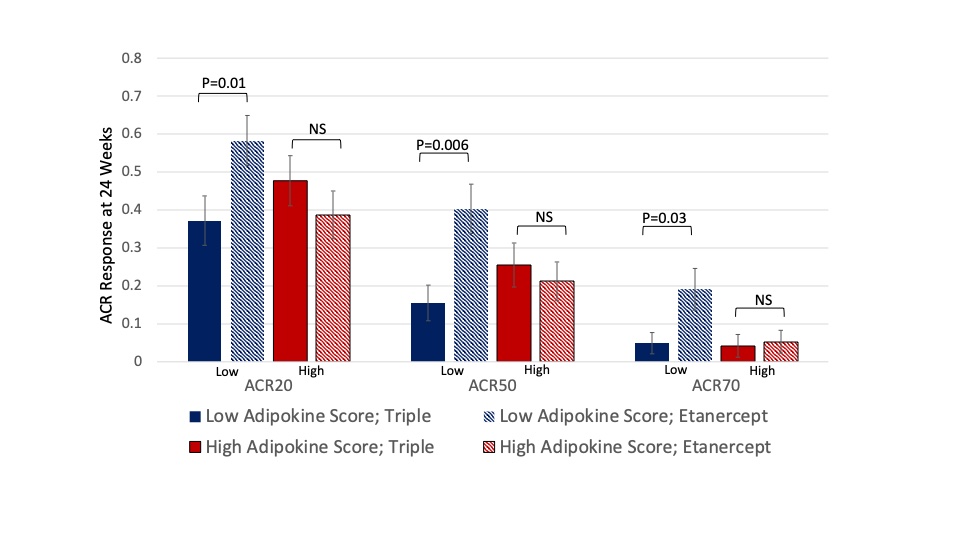
Results: We included 306 participants that were eligible from RACAT and 601 from TEAR. In RACAT, greater change in DAS28 and greater ACR20 response was observed for TNFi initiators compared to triple therapy among participants that were normal or underweight (ACR 20: 64% v. 23%) while no differences between TNFi and triple therapy were observed among overweight and obese (p for interaction = 0.001) (Table). Similar patterns were observed in TEAR, though were more modest. The pattern in TEAR was similar among methotrexate-naïve initiators at baseline and methotrexate inadequate responders at 6-months (combined data are shown in Table). In RACAT, adipokine scores below the median (< 2) and a profile consistent with lower adiposity were associated with greater response to TNFi (ACR20: 58% v. 37%) (Figure). The addition of adipokines to the model resulted in better model fit compared to a model with BMI alone (p=0.01).
Conclusion: Low BMI and evidence of low adiposity based on adipokine profiles are both associated with a superior response to initiation of TNFi compared to the initiation of triple therapy. This is the first study, to our knowledge, to identify a meaningful difference between two active strategies based on weight and body habitus and may reflect phenotypic differences or pharmacodynamic effects. The results support earlier escalation to TNFi among thin patients that do not respond to methotrexate and have implications for future clinical trial design.
To cite this abstract in AMA style:
Baker J, O'Dell J, England B, Giles J, Newcomb J, George M, Thiele G, Moreland L, Bridges S, Curtis J, Mikuls T. Differential Responses to Initial Treatment Strategies for Rheumatoid Arthritis Among Those with Lower Body Mass and Adiposity [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/differential-responses-to-initial-treatment-strategies-for-rheumatoid-arthritis-among-those-with-lower-body-mass-and-adiposity/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/differential-responses-to-initial-treatment-strategies-for-rheumatoid-arthritis-among-those-with-lower-body-mass-and-adiposity/