Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Down syndrome-associated arthritis (DA) is under-recognized with delay in diagnosis (1). The majority of those with DA present with polyarticular, rheumatoid factor (RF) and anti-nuclear antibody (ANA) negative disease, which is different compared to those with juvenile idiopathic arthritis (JIA). Therapy for JIA has been used to treat DA, but appear to be poorly tolerated, more toxic and less effective in patients with DA (1). The objective of this study was to characterize differences between those with DA and JIA, using the new Childhood Arthritis and Rheumatology Research Alliance Registry (nCARRA).
Methods: The nCARRA began prospectively collecting data on children with JIA in the United States and Canada in July 2015. Down syndrome (DS) is documented in the CARRA Registry as a coexisting condition. Between the dates of July 2015 and March 2019, patients with a diagnosis of JIA and DS were identified and matched (in a 1:5 ratio) on age, sex, and JIA subtype to patients with a diagnosis of JIA and without DS. Collected data included demographics, disease characteristics, laboratory results, treatment exposure, and outcomes measures.
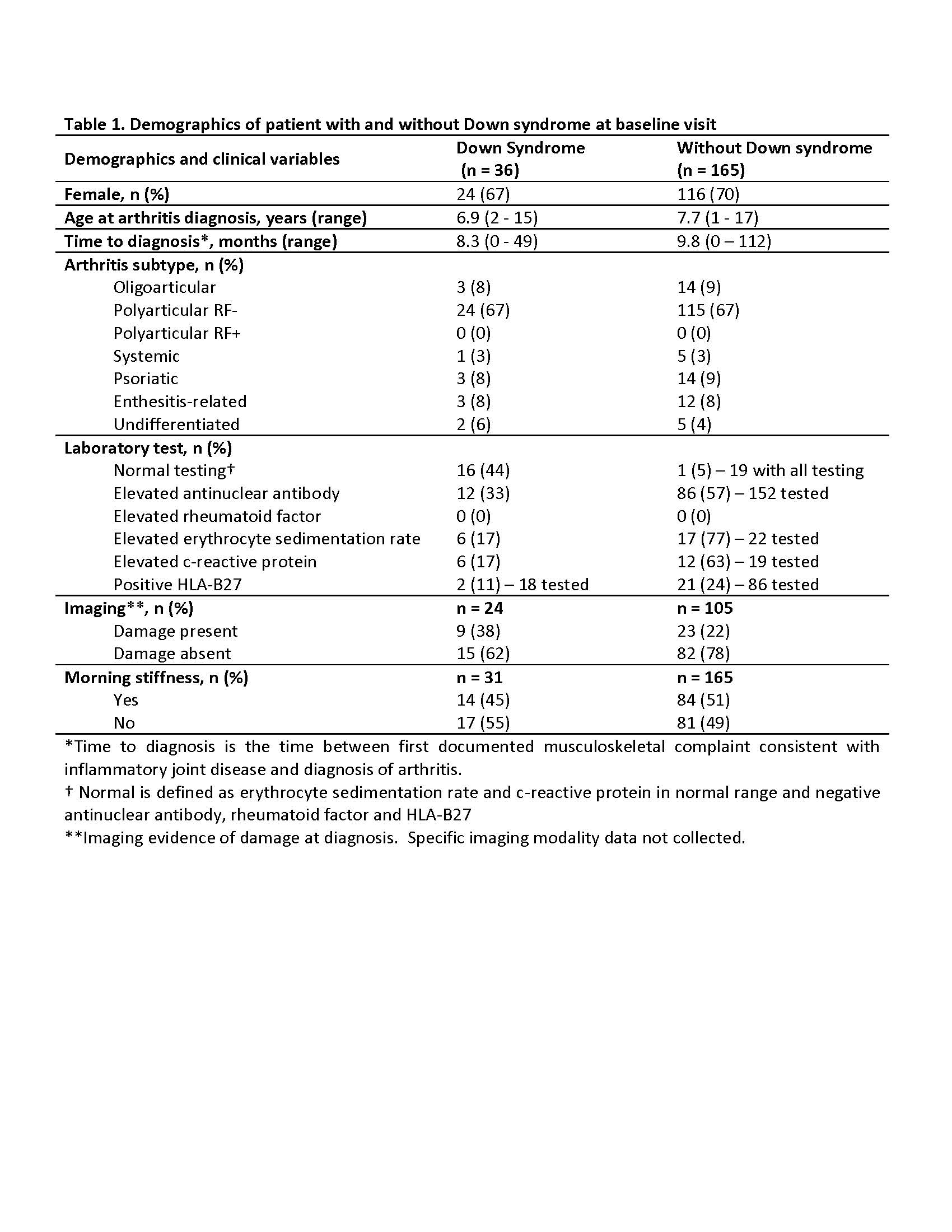
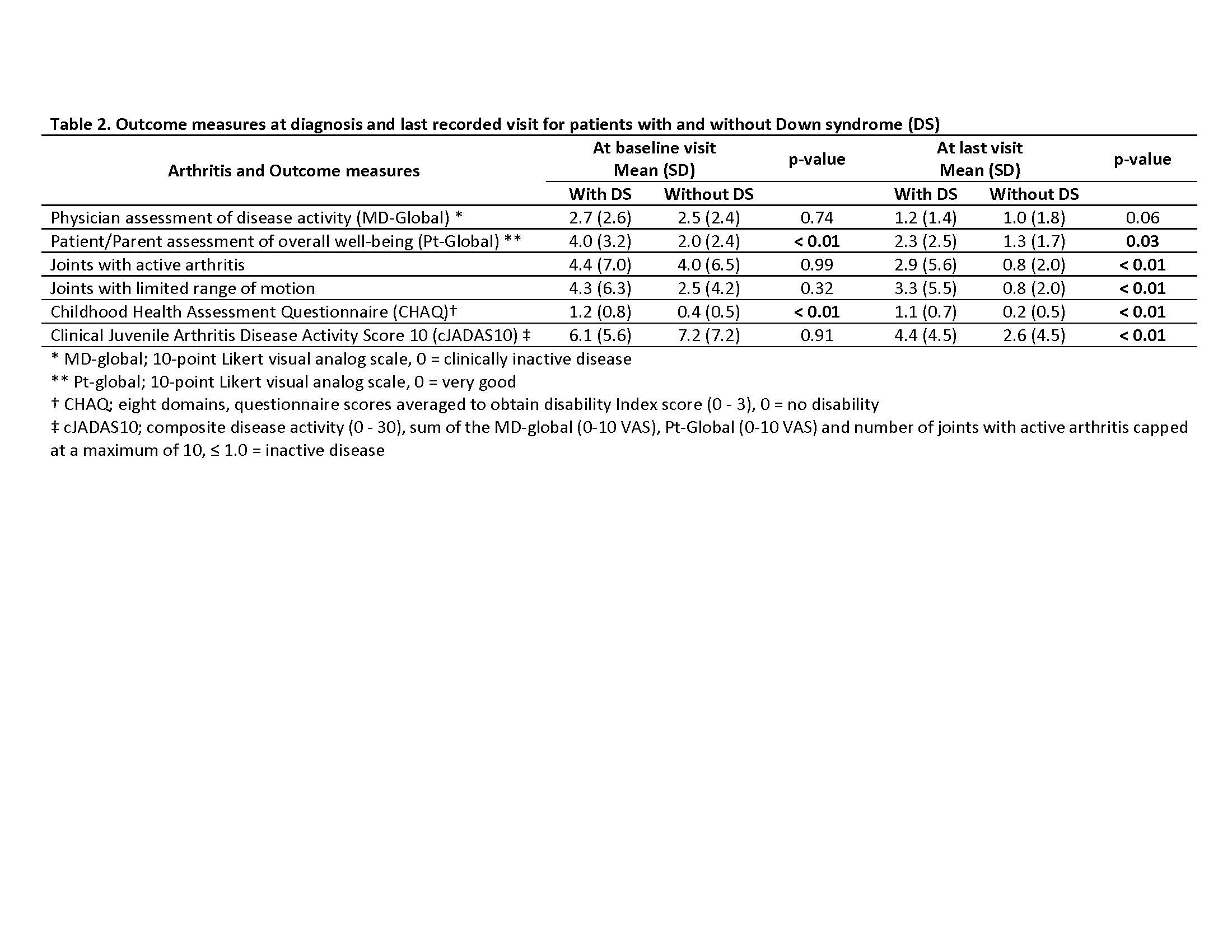
Results: Thirty-six patients were identified with DS and 165 patients without DS. Those with DS had a mean follow-up period of 4.5 years (SD 3.2), while those without had 4.7 years (SD 3.9). Most patients were female and had polyarticular, RF negative disease (Table 1.). The treatment approach at diagnosis for both groups were similar with no significant difference (p-value < 0.05) in initiation of disease modifying antirheumatic drug (DMARD)( 44% for those with DS, and 32% for those without DS) or biologic therapy (17% those with DS, and 7% for those without DS). DMARD therapy was used in 78% of those with DS and 87% of those without DS. Methotrexate was the most used DMARD for both groups, but those with DA had more DMARD adverse events (93% versus 25%). Biologic therapy was used in 75% of those with DS and 70% of those without DS, and biologic therapy ineffectiveness was much higher in those with DS (60% versus 17%). At entry into the nCARRA, there was no significant difference between joints with active arthritis, limited range-of-motion (ROM) or clinical juvenile arthritis disease activity score (cJADAS10), however, at last reported visit there was significant (p-value < 0.05) difference in almost all outcomes measures (Table 2.).
Conclusion: This study from a large multicenter registry reveals that children with DA have a similar presentation to children with JIA without DS, however, their disease course and disease burden is significantly worse. There is also more drug adverse events and higher rates of therapeutic ineffectiveness. This raises concern for possible physiologic reasons that place them at higher risk for resistant disease and treatment ineffectiveness. Collective work is needed to characterize DA, understand the pathophysiology, and understand clinical pharmacologic factors that will help identify the most effective and tolerated therapies to treat arthritis in children with Down syndrome.
To cite this abstract in AMA style:
Jones J, Smith C, Lovell D, Becker M. Differences and Similarities Between down Syndrome-associated Arthritis and Juvenile Idiopathic Arthritis in the New Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/differences-and-similarities-between-down-syndrome-associated-arthritis-and-juvenile-idiopathic-arthritis-in-the-new-childhood-arthritis-and-rheumatology-research-alliance-carra-registry/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/differences-and-similarities-between-down-syndrome-associated-arthritis-and-juvenile-idiopathic-arthritis-in-the-new-childhood-arthritis-and-rheumatology-research-alliance-carra-registry/