Session Information
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Traditional NSAIDs (tNSAIDs) are effective in treating arthritis pain but can be limited by GI adverse events and general bleeding related to platelet inhibition. Cyclooxygenase-2 (COX-2) selective NSAIDs (coxibs) were designed to avoid COX-1 inhibition and provide pain relief while avoiding hemorrhagic risk and GI side effects of tNSAIDs. In 2004, Merck voluntarily withdrew VIOXX (rofecoxib) following observations of increased risk of serious cardiovascular (CV) adverse events1, 2.
Methods: An extensive analysis of the published data regarding the CV safety, pharmacologic profile, and regulatory and commercialization history of rofecoxib was conducted.
A systematic review of the CV data for tNSAIDs and coxibs has subsequently demonstrated that CV risk is a dose and duration-related class effect for all NSAIDs3,4. This CV risk is recognized as a class effect by FDA, with class labeling for all NSAIDs5. Why then, did rofecoxib appear to be an outlier for CV risk?
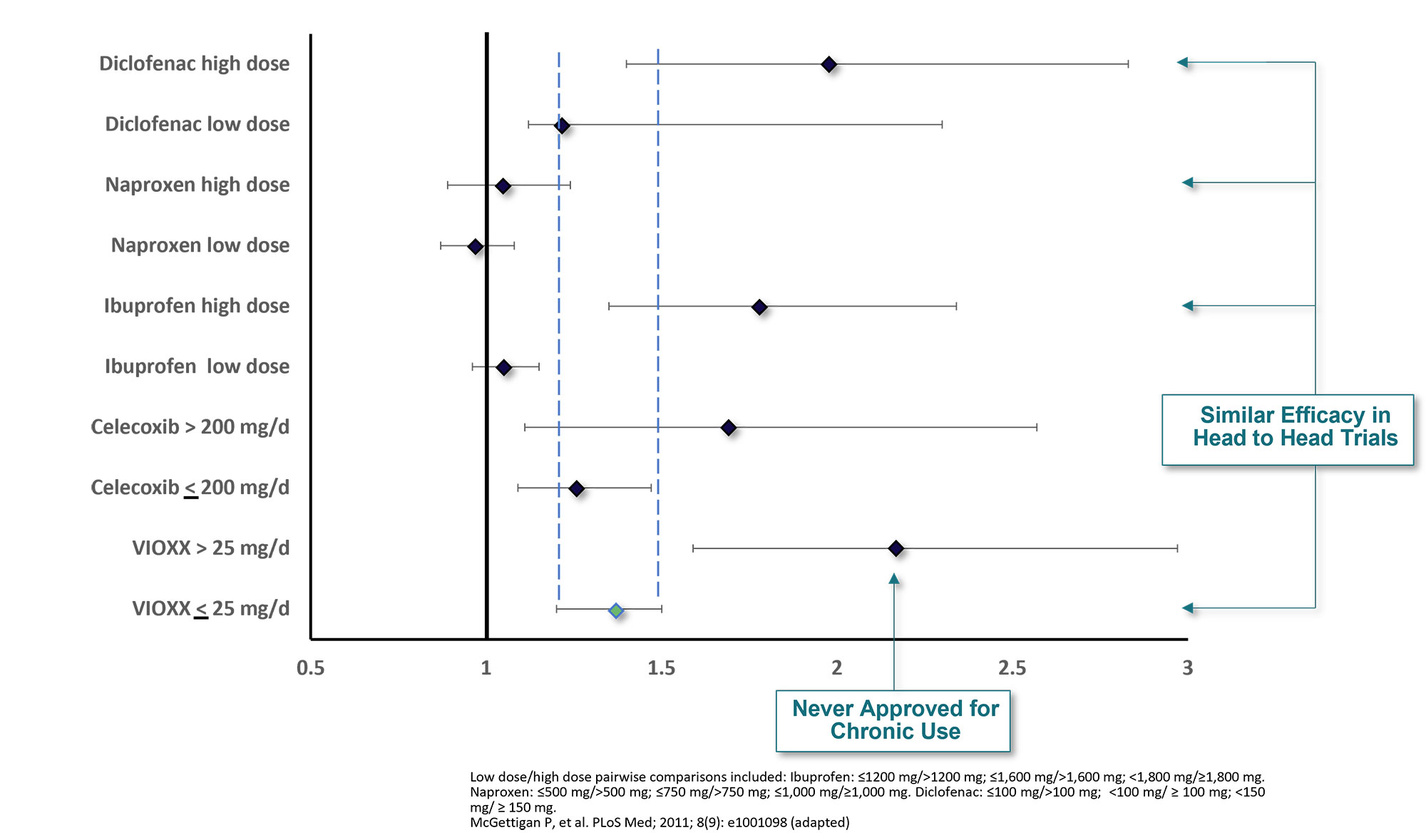
Results: Rofecoxib was approved for chronic use at 12.5 mg and 25 mg for OA and at 25 mg for RA; the 50-mg dose of rofecoxib was indicated only for acute pain based on studies with durations up to 5 days and was not recommended for chronic use.
Rofecoxib’s unique profile among the NSAIDs included its tolerability at high doses, high bioavailability and once daily dosing. This profile, along with 30-day sample packs for the 50-mg dose (the smallest commercially available pack-size was a 30-count bottle) led to the chronic use of the acute pain dose; 17% of prescriptions were for >25 mg/day6. Evaluations of dose-related CV effects for rofecoxib compared with other NSAIDs showed that doses >25 mg to be the outlier (Figure 1). Rofecoxib doses ≤25 mg/day had a similar CV risk as equi-efficacious doses of ibuprofen, diclofenac, and celecoxib; >25mg/day had 2X the risk7. It appears that chronic off-label use of VIOXX 50 mg led to higher rates of CV events for VIOXX relative to other NSAIDs.
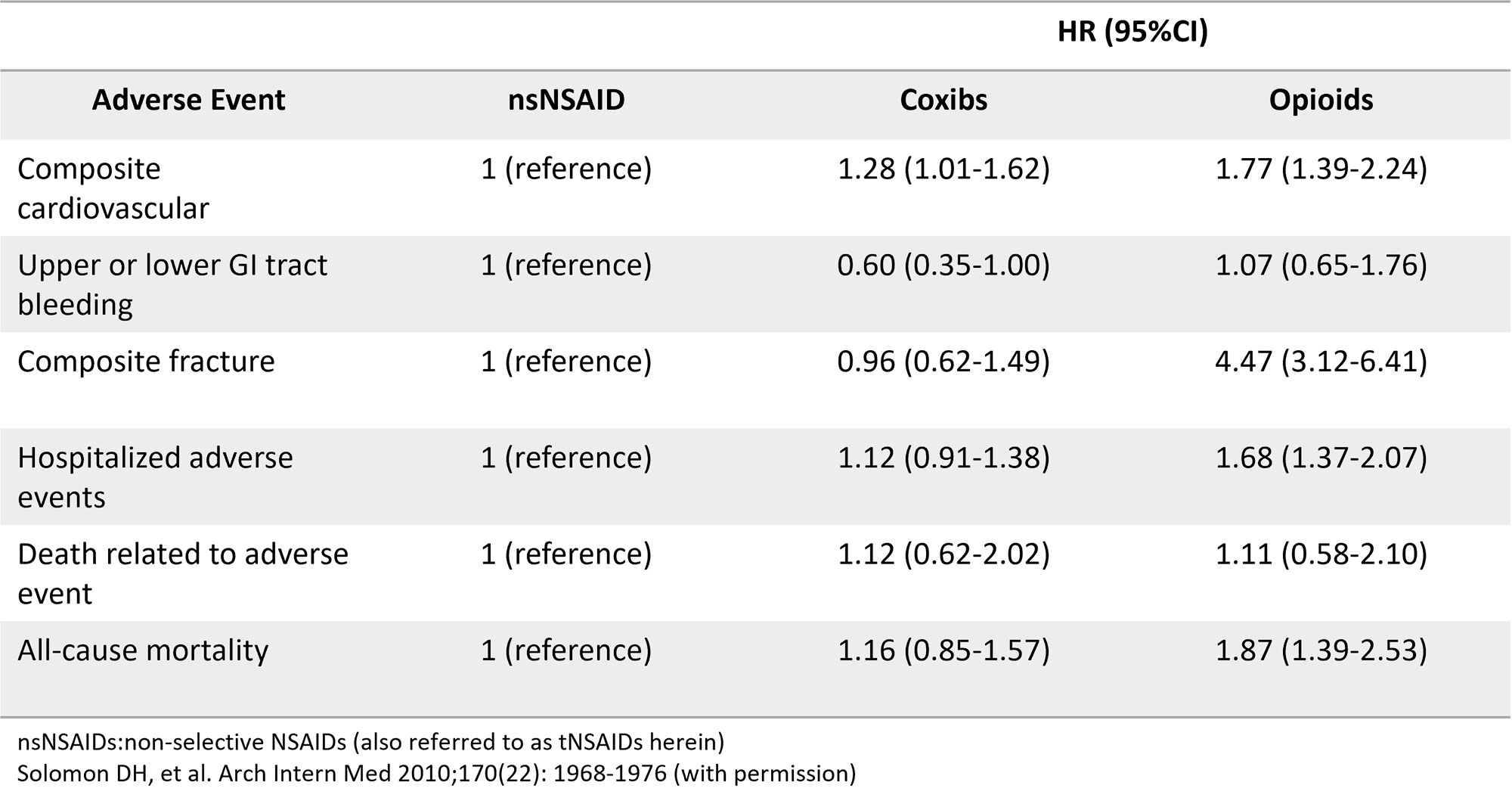
The withdrawal of rofecoxib limited the non-opioid treatment options for people who cannot take or who do not tolerate tNSAIDs. The use of opioids for arthritic conditions has risen markedly since 20048. Beyond addiction, opioid treatments carry their own significant safety risks that compare unfavorably to coxibs on nearly every metric (Table 1)9.
Conclusion: Given its unique profile, rofecoxib ≤25 mg/day may be a viable treatment option in patients populations for whom tNSAIDs are avoided and where opioids are frequently used. Tremeau Pharmaceuticals is currently conducting a pivotal Phase III trial evaluating rofecoxib in the treatment of hemophilic arthropathy (NCT04684511) and is pursuing other indications.
References:
1. Bombardier C, et al. NEJM 2000;343(21):1520-1528
2. Bresalier RS, et al. NEJM 2005;352(11):1092-1102
3. CNT Collaboration Lancet 2013;382(9894):769-779
4. McGettigan P, et al. PLoS Med; 2011; 8(9):e1001098
5. US FDA. 2015 FDA Drug Safety Communication
6. Griffin MR, et al. Pharmacoepidemiol Drug Saf 2004;13(6):339-43
7. Baron JA, et al. Lancet 2008; 372(9651):1756-1764
8. Santo L, et al. Arthritis Care & Res 2021;73(10):1430-1435
9. Solomon DH, et al. Arch Intern Med 2010;170(22): 1968-1976
with arthritis initiating prescription analgesic treatment
To cite this abstract in AMA style:
Boice J, Corrigan M, Sippy B. Diagnosing the Cardiovascular Risk of VIOXX: A Place for Rofecoxib in Pain Management [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/diagnosing-the-cardiovascular-risk-of-vioxx-a-place-for-rofecoxib-in-pain-management/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/diagnosing-the-cardiovascular-risk-of-vioxx-a-place-for-rofecoxib-in-pain-management/