Session Information
Date: Monday, November 13, 2023
Title: (1365–1382) Sjögren’s Syndrome – Basic & Clinical Science Poster I
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Sjögren’s is a heterogenous chronic auto-immune disease, characterized by excessive dryness of the eyes and mouth, as well as systemic complications which can significantly impact patients’ health-related quality of life (HRQoL). Existing patient-reported outcome (PRO) measures focus on the frequency/severity of symptoms but do not capture the impacts that Sjögren’s has on patients’ HRQoL. To address this gap, the Sjögren’s-Related Quality of Life (SRQoL), was developed in line with Food and Drug Administration’s Clinical Outcome Assessment (COA) guidance documents.1-4 Qualitative interviews were conducted to evaluate the content validity and appropriateness of the SRQoL.
Methods: Patient advisory boards, social media listening, previous patient interviews, and findings from a prior literature review were reviewed to gain insights into the patient experience and HRQoL impact of Sjögren’s to develop the SRQoL. Global impression of severity (PGI-S) and change (PGI-C) items were developed to inform further validation of the SRQoL. SRQoL and PGI-S/C development involved a patient advocate and physician as research partners. Combined qualitative concept elicitation (CE) and cognitive debriefing (CD) interviews are being conducted with adult (≥18 years) patients with primary Sjögren’s from the US and UK, over two rounds. Round-one has been completed (n=10; 70% female; mean age = 52 years).
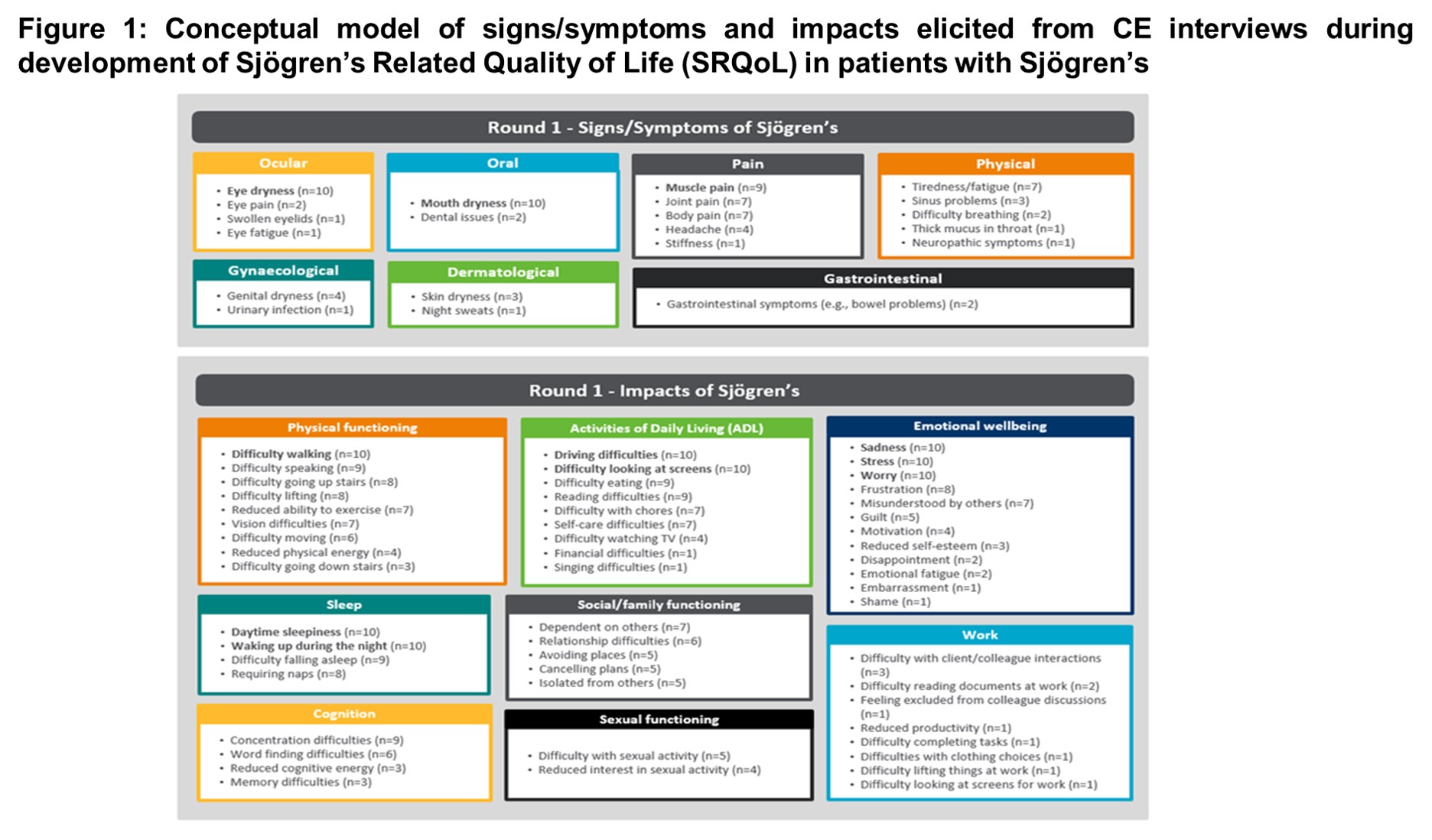
Results: From the review, an initial 36-item draft PRO measure was developed to assess HRQoL across seven hypothesized domains including physical functioning, activities of daily living, cognition, sleep, emotional wellbeing, social/family functioning, and work. Round-one CE results were consistent with Sjögren’s related symptoms and HRQoL impacts elicited from the review (difficulty walking, driving difficulties, low mood/sadness, and daytime sleepiness). Concepts from the participant interviews (round one) were organized into a preliminary conceptual model in Figure 1. SRQoL and PGI-S/C items were well understood and relevant to most participants during round-one CD. Participants endorsed the 7-day recall period and frequency and severity response scales.
Conclusion: Initial findings support the content validity and suitability of the draft SRQoL, with round-two interviews currently underway. Following further testing and psychometric evaluation, the SRQoL is intended to provide greater understanding of patient burden and treatment benefit of Sjögren’s in randomized clinical trials and may be used in clinical practice to monitor patients’ HRQoL and improve patient-physician communication.
References:
- FDA PFDD: Collecting Comprehensive and Representative Input, 2018
- FDA PFDD: Methods to Identify What Is Important to Patients, 2022
- FDA PFDD: Selecting, Developing, or Modifying Fit-for-Purpose Clinical Outcome Assessments, 2022
- FDA PFDD: Incorporating Clinical Outcome Assessments Into Endpoints for Regulatory Decision-Making, 2023
To cite this abstract in AMA style:
A Fisher B, Stone L, Marvel J, Goswami P, Steenackers M, Kenney G, Perella C, Hueber W, Howse C, Gargon E, Chohan A, Mayhew M, Williamson N. Development of the Sjögren’s-related Quality of Life (SRQoL) to Assess Health-related Quality of Life (HRQoL) in Sjögren’s [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/development-of-the-sjogrens-related-quality-of-life-srqol-to-assess-health-related-quality-of-life-hrqol-in-sjogrens/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/development-of-the-sjogrens-related-quality-of-life-srqol-to-assess-health-related-quality-of-life-hrqol-in-sjogrens/