Session Information
Session Type: Abstract Session
Session Time: 1:00PM-2:30PM
Background/Purpose: Severe allopurinol hypersensitivity (SAH), although uncommon, is a serious adverse event of allopurinol. Both genetic (HLA-B*58:01) and non-genetic factors (female gender, age ≥60-65 years, renal impairment, high starting dose of allopurinol and use of diuretics including hydrochlorothiazide and furosemide) have been contributed to SAH. However, HLA-B*58:01 testing is costly, and not available in many institutions. This study aimed to develop a model to predict of SAH using non-genetic clinical risk factors.
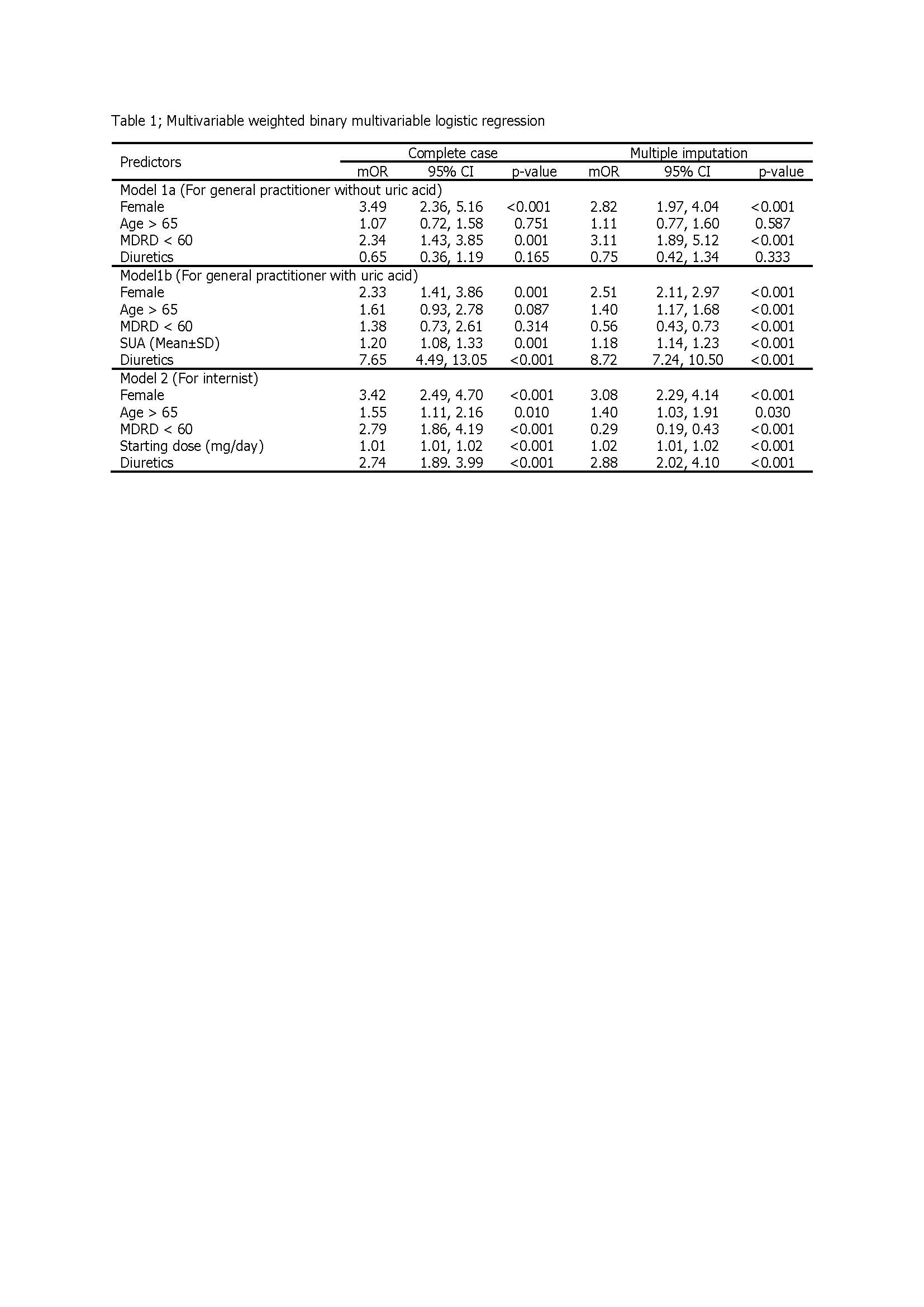
Methods: The study was a retrospective observational study using an incidence density sampling. SAH cases were collected from tertiary care medical center; where most non-SAH cases were collected from primary care hospitals in 4 provinces, and 717 cases also from a tertiary care medical center. The data of non-genetic factors including gender, age, renal function, concomitant use of diuretics, (hydrochlorothiazide or furosemide), starting dose of allopurinol and serum uric acid (SUA) of non-SAH cases selected only the first description of allopurinol. The binary prevalence-weighted logistic regression analysis was used to develop the prediction models for SAH. Three models were developed following real general practice.
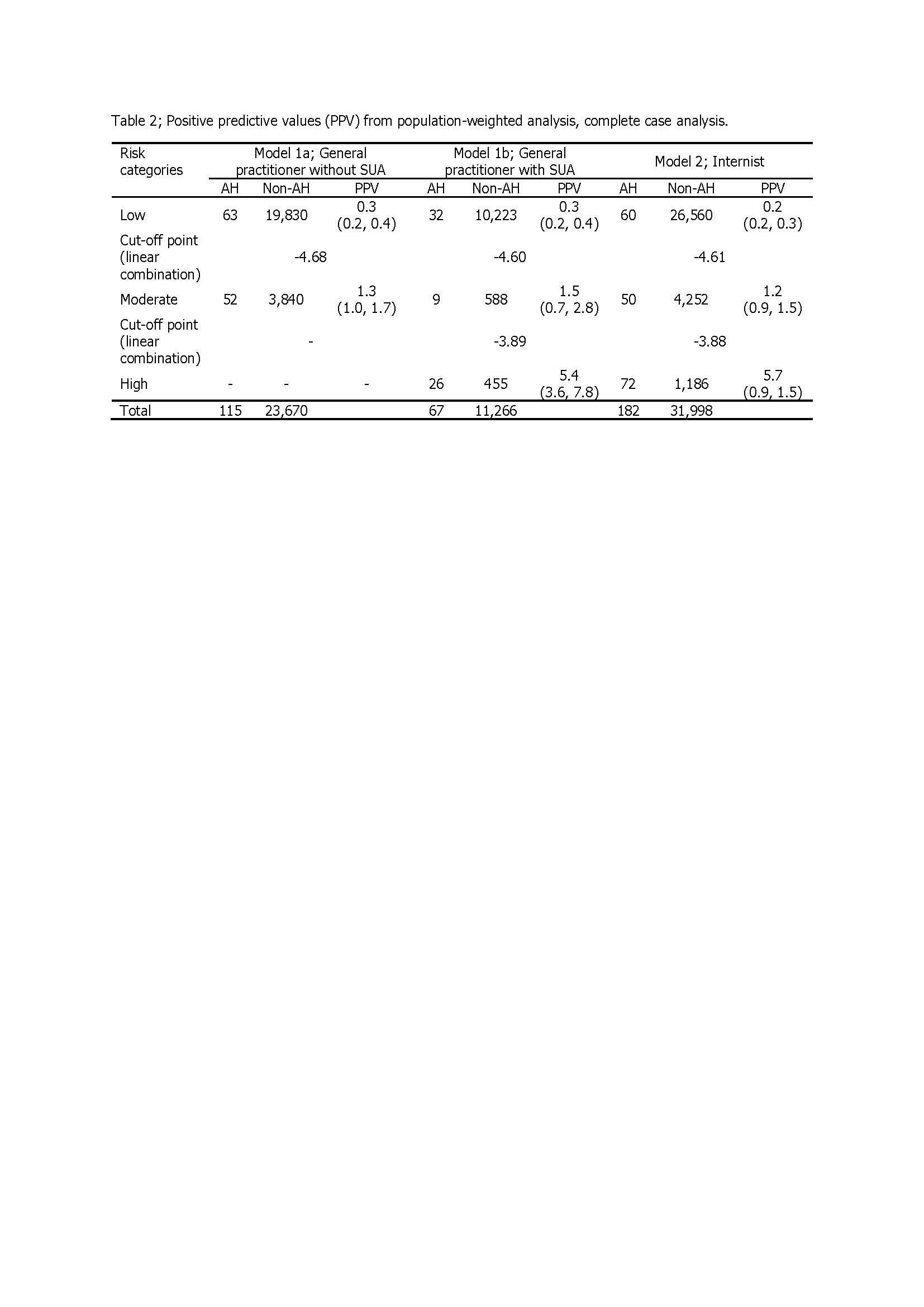
Results: Totally, there were 209 cases of SAH and 23,068 cases of non-SAH (prevalence 0.89%). Factors associated with the development of SAH within 90 days of starting allopurinol were female gender, age ≥ 65 years, renal impairment, high starting dose of allopurinol, concomitant use of hydrochlorothiazide or furosemide, and high SUA level prior to prescription. Model 1a and model 1b were applied for patients who did not have and had SUA level when starting allopurinol, respectively. Model 2 was applied for patients who had all non-genetic risk factors, started allopurinol within 60 days, and did not have SAH events. The area under the receiver operating characteristic curve (AuROC) (95%CI) for model 1a, model 1b and model 2 were 0.72 (0.67, 0.76), 0.81 (0.75, 0.86) and 0.82 (0.79, 0.86), respectively. The performance for the prediction SAH of each model was good. The score of 0-1%, 1-2% and 2-100% predicted low, moderate and high risk for development of SAH after starting allopurinol, respectively.
Conclusion: Model 1a and model 1b can predict SAH from patients who had their first prescribed allopurinol, and model 2 can predict SAH for the patients who had been taken allopurinol within 60 days but no SAH. The scoring system of each model can help clinician for properly prescribe allopurinol in real clinical practice before SAH being developed. Allopurinol can be started safely in the low risk group, but might a close monitoring in the moderate risk group. In the high risk group, allopurinol should not be used, and the other urate lowing therapy should be considered. However, the genetic factors that had effect to SAH were not considered in this study.
To cite this abstract in AMA style:
Towiwat P, Tassaneeyakul W, Lawanaskol S, Sukasem C, Nakkam N, Rerkpattanapipat T, Klaewsongkram J, Rerknimitr P, Poolpun D, Louthrenoo W, Saksit N. Development of Non-genetic Risk Stratification Model of Severe Allopurinol Hypersensitivity Syndrome, a Multicenter Study in Thailand [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/development-of-non-genetic-risk-stratification-model-of-severe-allopurinol-hypersensitivity-syndrome-a-multicenter-study-in-thailand/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/development-of-non-genetic-risk-stratification-model-of-severe-allopurinol-hypersensitivity-syndrome-a-multicenter-study-in-thailand/