Session Information
Date: Sunday, October 26, 2025
Title: (0671–0710) Systemic Sclerosis & Related Disorders – Clinical Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Inflammatory joint and/or tendon involvement affects up to 30% of patients with systemic sclerosis (SSc), resulting in pain, reduced joint function, and impaired patients’ quality of life. The lack of standardized outcome measures for assessing articular activity in SSc hampers therapeutic development and data comparability. To bridge this gap, we aimed to develop a consensus-based composite score to assess articular activity in adult SSc, suitable for research settings and/or clinical practice.
Methods: A steering committee was convened, consisting of 12 rheumatologists, 1 epidemiologist, 1 occupational therapist, and 3 patient research partners. The development of the articular activity score followed a stepwise process (Figure 1). First, a scoping review was conducted using PubMed (January 1960 to April 2023); two reviewers (B.B. and M.E.) independently screened studies on joint/tendon involvement in SSc and extracted assessment tools. Following expert discussions, instruments demonstrating at least 70% validity and feasibility after the committee voting were further evaluated by two independent reviewers according to OMERACT (Outcome Measures in Rheumatology) guidelines. These findings were then presented to the steering committee, and a two-round online Delphi survey was used to finalize the core set.
Results: Of the 770 references initially identified, 658 were excluded based on title or abstract screening, leaving 112 articles for full-text review; ultimately, 86 studies met the inclusion criteria. From these, 46 instruments that were used in at least two studies were identified. Based on domain validity and feasibility, 9 clinical, 1 serological, 3 composite scores, and 3 patient-reported outcome measures were shortlisted for further evaluation (Table 1). Following an assessment of measurement properties according to OMERACT guidelines, the steering committee voted and excluded 10 items due to missing specificity or feasibility on joint activity assessment. 6 final instruments were selected for inclusion in a composite outcome measure to assess articular activity in patients with SSc. The selected instruments covered clinical (28-joint tender/swollen joint count with the addition of distal interphalangeal joints; presence of tendon friction rubs), serologic (CRP) and patient/physician reported outcomes (VAS activity pain patient, VAS activity doctor) domains.
Conclusion: International, multi-disciplinary SSc experts and patient partners have identified 6 feasible core instruments to assess articular activity in SSc patients, covering both joint and tendon involvement. This core set provides the basis to further develop a composite score for articular involvement in SSc for use in clinical practice and clinical trials. Future steps will include performance validation within longitudinal SSc cohorts.
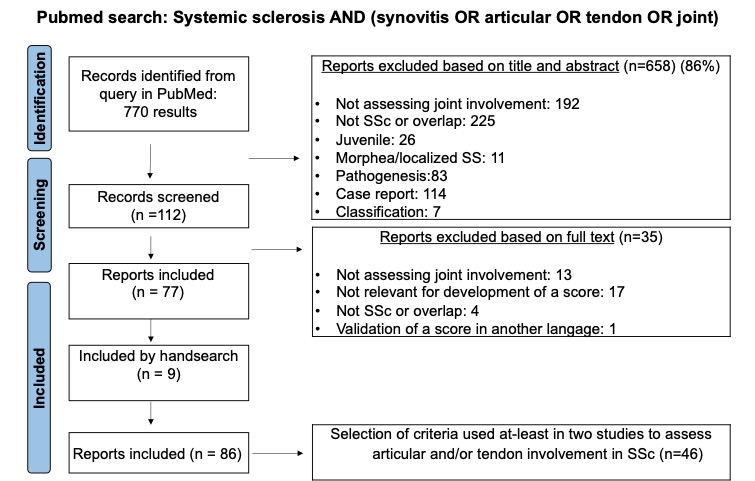 Figure 1: Scoping review search strategy.
Figure 1: Scoping review search strategy.
.jpg) Table 1: Identified core instruments for assessing disease articular activity in SSc patients. The 28-joint count includes the wrists, metacarpophalangeal (MCP) joints, proximal interphalangeal (PIP) joints, elbows, shoulders, and knees. The 50-joint count builds on the 28-joint count by also including the distal interphalangeal (DIP) joints, hips, ankles, and metatarsophalangeal (MTP) joints.
Table 1: Identified core instruments for assessing disease articular activity in SSc patients. The 28-joint count includes the wrists, metacarpophalangeal (MCP) joints, proximal interphalangeal (PIP) joints, elbows, shoulders, and knees. The 50-joint count builds on the 28-joint count by also including the distal interphalangeal (DIP) joints, hips, ankles, and metatarsophalangeal (MTP) joints.
To cite this abstract in AMA style:
Burja B, Welsing P, Lescoat A, Eisenring A, Hoffmann-Vold A, Leroy David C, Khanna D, Del Galdo F, Iudici M, Pope J, Spierings j, Vonk M, Truchetet M, Clergeau M, Hughes M, Murphy S, Frech T, Distler O, Elhai M. Development of an Articular Activity Score in Systemic Sclerosis (ASSESS): Identifying Core Instruments for Disease Activity Assessment [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/development-of-an-articular-activity-score-in-systemic-sclerosis-assess-identifying-core-instruments-for-disease-activity-assessment/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/development-of-an-articular-activity-score-in-systemic-sclerosis-assess-identifying-core-instruments-for-disease-activity-assessment/
