Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: For RA patients, regular monitoring of disease activity is essential, but in-person assessments may not always be feasible due to the increasing pressure on our healthcare system. One solution might be the implementation of ‘patient-reported outcome measures’ (PROMs) to remotely monitor the disease activity. Previous research combining multiple PROMs showed moderate diagnostic accuracy (‘Area Under the Receiver Operating Characteristic curve’ (AUC-ROC) of 0.76) in detecting a disease flare. However, these individual PROMs may include items unrelated to disease activity, hindering optimal discrimination.Therefore, our aim was to develop a model composed of a selection of independent PROM-items, that accurately represents the disease activity score in patients with RA.
Methods: Data from early and established RA patients in the ‘treatment in the Rotterdam Early Arthritis Cohort’ and ‘TApering strategies in Rheumatoid Arthritis’ trials were used and randomly split (1:1) into development and internal validation cohorts. For external validation data from RA patients in the ‘Leiden Early Arthritis Clinic’ were used. Patients were included if they met the 1987 or 2010 RA criteria and had at least one PROM-item and the DAS44 measured at the same time point.With ‘Least Absolute Shrinkage and Selection Operator’ regression, a model was developed using age, sex, disease duration, and individual PROM-items (HAQ-items, pain (numeric rating scale (NRS) 0-10), general health (visual analogue (VAS) 0-100 millimeters (mm)), and fatigue (VAS 0-100mm)) to predict the DAS44. The final model was evaluated for its ability to detect active disease (DAS44 >2.4) using the AUC-ROC. Sensitivity and specificity where then assessed across thresholds based on: (I) the Youden index, (II) DAS44 >2.4, and thresholds for (III) 95% sensitivity and (IV) 95% specificity in the development cohort.
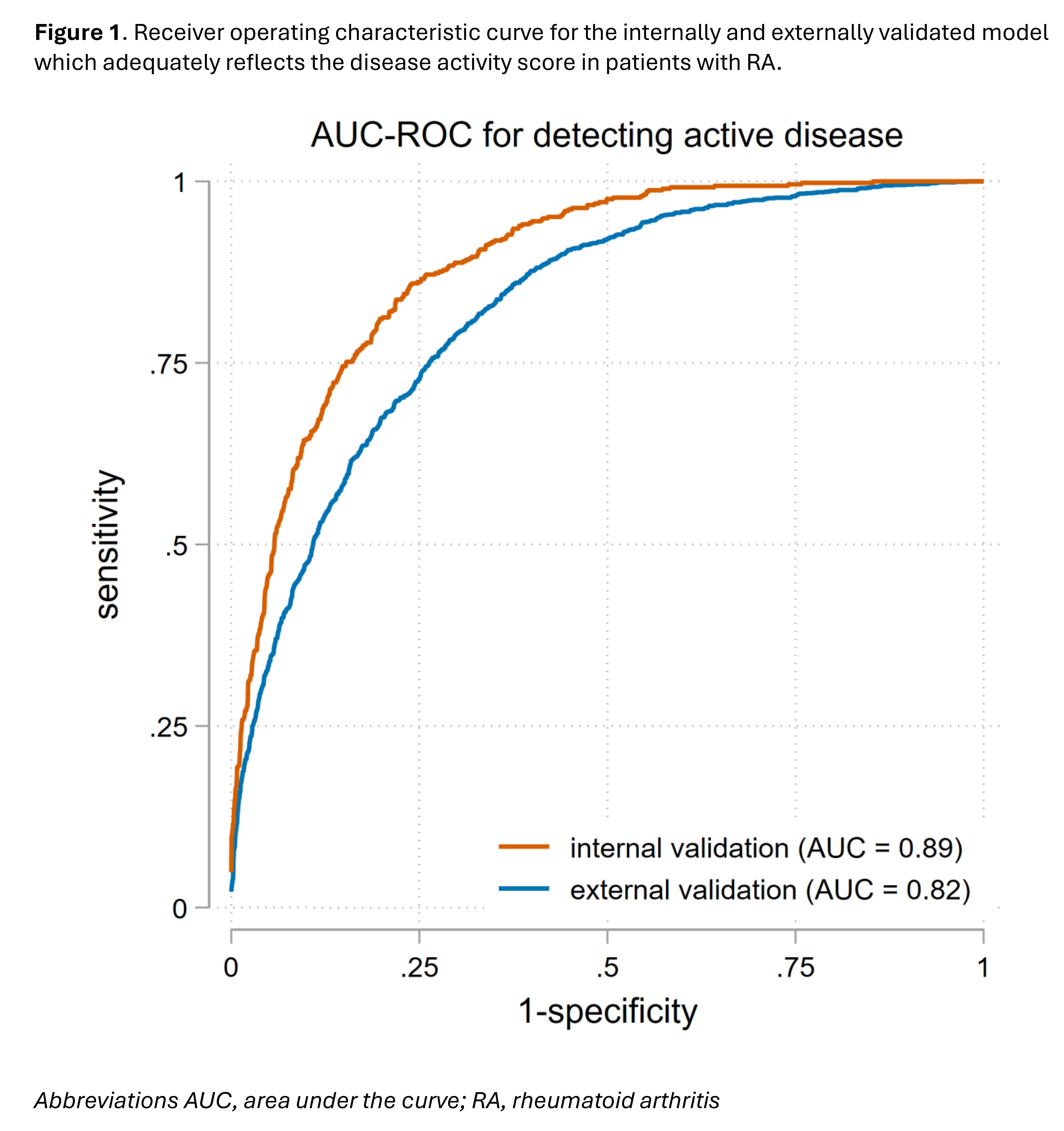
Results: The development and internal validation cohorts included, 423 early and 187 established RA patients with 5802 visits; active disease was present in 20% of these visits. The external validation cohort included 793 patients with 2467 visits, and in 38% of those visits active disease was present. The final model included 12 of the 26 potential variables (age, sex, disease duration, 7 HAQ-items, NRS pain, and VAS general health) and showed an excellent discriminative ability (AUC-ROC=0.89 for internal validation, 0.82 for external validation) (see figure 1). Table 1 summarizes the sensitivity and specificity for the different thresholds. Using the Youden index (1.93), the sensitivity for detecting active disease is 78% and the specificity is 82%.
Conclusion: A combination of age, sex, disease duration, VAS general heath, NRS pain and seven HAQ-items is able to adequately differentiate between well-controlled and active disease with excellent diagnostic accuracy, even after validation in an external dataset. This could support remote monitoring of RA patients.
To cite this abstract in AMA style:
Looijen A, Welsing P, Bergstra S, van der Helm-van Mil A, de Jong P. Development and Validation of an Accurate Patient Reported Outcome Measure-based Disease Activity Score to Enable Remote Monitoring in RA [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/development-and-validation-of-an-accurate-patient-reported-outcome-measure-based-disease-activity-score-to-enable-remote-monitoring-in-ra/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/development-and-validation-of-an-accurate-patient-reported-outcome-measure-based-disease-activity-score-to-enable-remote-monitoring-in-ra/

.jpg)