Session Information
Date: Monday, November 9, 2020
Title: Pediatric Rheumatology – Clinical Poster III: SLE, Vasculitis, & JDM
Session Type: Poster Session D
Session Time: 9:00AM-11:00AM
Background/Purpose: Corticosteroids (CS) remain the mainstay of therapy for childhood-onset systemic lupus erythematosus (cSLE). However, widely accepted strategies for oral (PO) or intravenous (IV) CS dosing are lacking. We aimed to 1) develop a standardized CS dosing regimen (SSR) and 2) achieve consensus for this SSR among pediatric rheumatology and nephrology physicians treating cSLE, including lupus nephritis (LN).
Methods: Consensus formation techniques were used. A Delphi questionnaire was completed to select relevant covariates influencing CS dosing in LN (Step 1). Retrospective data from 147 children with proliferative LN at 8 major cSLE treatment sites in North America were used to generate Patient Profiles (PP) describing cSLE course at 2 subsequent visits (Step 2). PP were sent to 142 physicians experienced in cSLE to rate the course of LN and extrarenal disease and propose PO and IV CS dose adjustments (Step 3). Using PP data for which consensus was achieved, an SSR was developed (Step 4) and refined based on responses from another questionnaire and a focus group of experienced physicians (Step 5). Then, the SSR was validated (Step 6) using a second subset of PP describing disease course for up to 6 months since the time of initial kidney biopsy. Consensus was defined as agreement by majority ( >50%) of PP ratings (Step 3, Step 6).
Results: In Steps 1 and 3, 103 physicians answered Delphi questions and rated 353 PP (response rate: 73%). In Step 6, 18 physicians (mean 13.4 years of experience) were asked to review 33 PP each resulting in 564 PP ratings, of which 437 (77.5%) and 460 (81.6%) yielded consensus on PO and IV CS dosing respectively. PO and IV CS dosing depends on the patient’s weight, the course of extrarenal activity, measured by the extrarenal SLEDAI score (Figure 1d), and the course of LN, described by changes/status of 3 LN response variables (LN-RVs, Figure 1a-c). The SSR reflects dosing customs agreed upon by physicians. Table 1 summarizes the SSR with focus on 2 disease courses (1:stable extrarenal/various LN; 2:stable LN/various extrarenal), with permutations of extrarenal disease course (much worse, mild-moderately worse, active stable/improved, inactive) and LN course (flare, mild-moderately worse, active stable/improved/partial or complete renal remission). Doses of PO CS ≥ 40 mg are guided by LN course except in major extrarenal flares with potential organ damage. IV CS are used for worsening disease courses that fail to respond to PO CS of ≥ 40 mg up to 4 weeks (Table 1). Small decreases of PO CS occur even with stable LN or extrarenal activity. Complete renal remission allows more pronounced reduction of PO CS. Beyond 6 months post kidney biopsy (maintenance therapy), CS dosing is informed by the renal response during induction therapy, the course of LN, and extrarenal activity (Table 1).
Conclusion: The proposed standardized CS dosing regimen for LN in cSLE may be useful for clinical care and regulation of background CS use during clinical trials of new medications for cSLE.
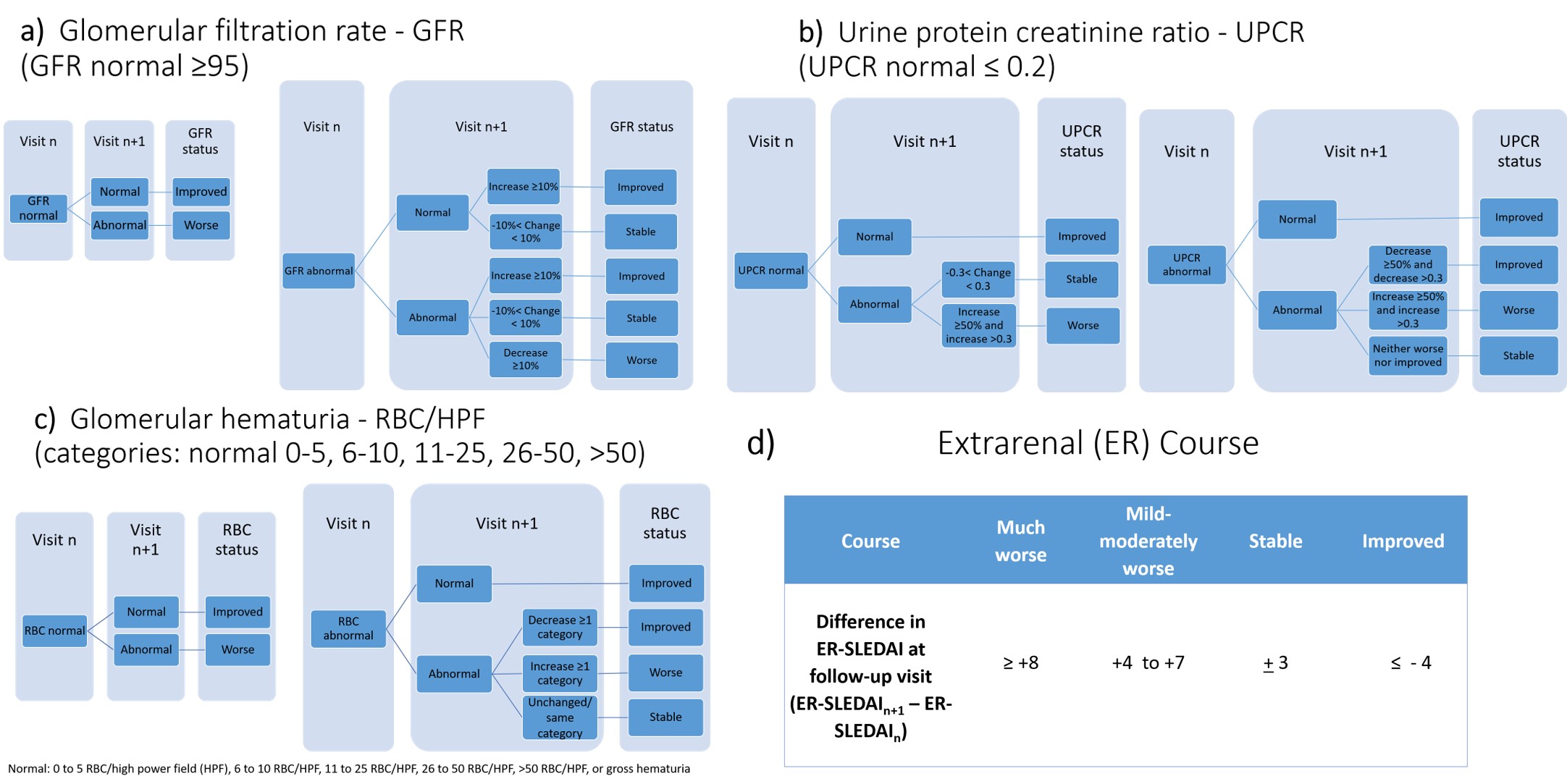 Figure 1. Consensus definitions for assessing changes in lupus nephritis response variables (LN-RVs) and extrarenal (ER) disease activity between clinical encounters
Figure 1. Consensus definitions for assessing changes in lupus nephritis response variables (LN-RVs) and extrarenal (ER) disease activity between clinical encounters
 Table 1. Corticosteroid (CS) use provided by the standardized CS dosing regimen (SSR)
Table 1. Corticosteroid (CS) use provided by the standardized CS dosing regimen (SSR)
To cite this abstract in AMA style:
Chalhoub N, Rouster-Stevens K, Klein-Gitelman M, Onel K, Goilav B, Savani S, Ruth N, Qiu T, Aljaberi N, Deng J, Merritt A, Laskin B, Sagcal-Gironella A, Ardoin S, Levy D, Wenderfer S, Huang B, Brunner H, Project Investigators L. Developing a Standardized Corticosteroid Dosing Regimen in Pediatric Proliferative Lupus Nephritis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/developing-a-standardized-corticosteroid-dosing-regimen-in-pediatric-proliferative-lupus-nephritis/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/developing-a-standardized-corticosteroid-dosing-regimen-in-pediatric-proliferative-lupus-nephritis/
