Session Information
Date: Sunday, November 13, 2022
Title: Pediatric Rheumatology – Clinical Poster II: Connective Tissue Disease
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
Background/Purpose: Juvenile localized scleroderma (jLS) is an inflammatory and fibrosing disease that causes severe morbidity (e.g., hemiatrophy, arthropathy, seizures), with functional impairment found in >25% of patients. Accurate tracking of disease activity is essential as controlling inflammation is the best current strategy to minimize damage. Tools that enable accurate tracking of disease activity are thus crucial for improving long-term outcome. One measure, the Computerized Skin Score (CSS), was used in a placebo-controlled randomized clinical trial to demonstrate the efficacy of methotrexate in jLS (Zulian et al Arthritis Rheum 2011;63:1998). The CSS involves tracing the boundaries of an active skin lesion onto a Tegaderm sheet, then scanning this into a computer programmed to calculate the surface area. The CSS, however, has not been widely used because of the need for special materials and set up, and time involved (mean 5.35 minutes/lesion). We developed a mobile app to facilitate routine tracking of active skin disease extent.
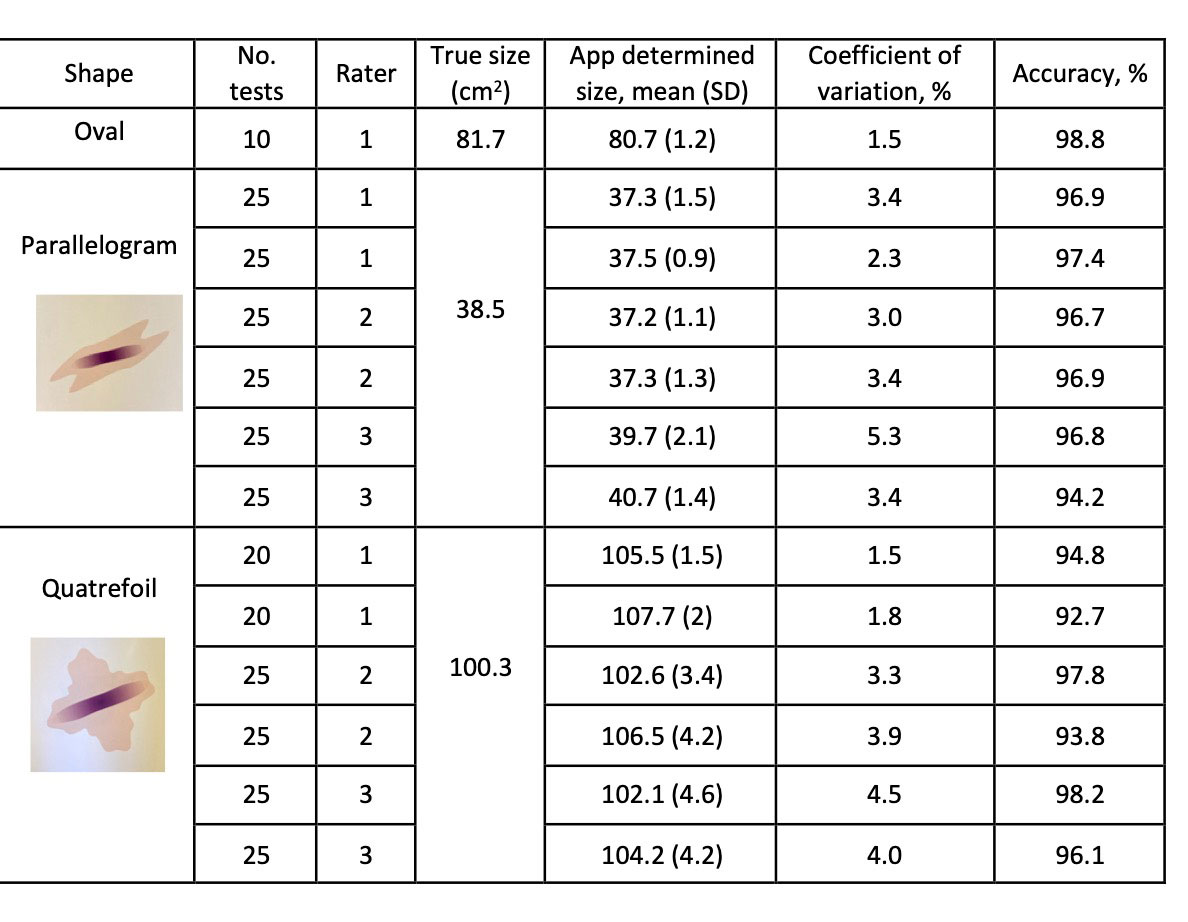
Methods: The app was developed for a Samsung galaxy tablet using EmguCV package on Microsoft Xamarin platform. Boundary detecting algorithm and stylus function were built in. The tablet’s camera is used to capture the lesion image. The user traces the boundaries of the uploaded lesion image with the stylus, and the app calculates its area by comparing the number of pixels in the lesion to that of a standard. Testing of the app was done on Mathematica generated shapes of known sizes. Reproducibility of area calculation was examined by having each rater determine the area of test shapes multiple times. For 2 shapes, two runs were conducted by each of three raters, with each run consisting of 20 to 25 independent app determinations. The standard deviation and coefficient of variation were calculated for each run. The accuracy of size determination was calculated by comparing the app determined size to the true size of the test shape (100- 100*[true size-observed size]/true size) for each run.
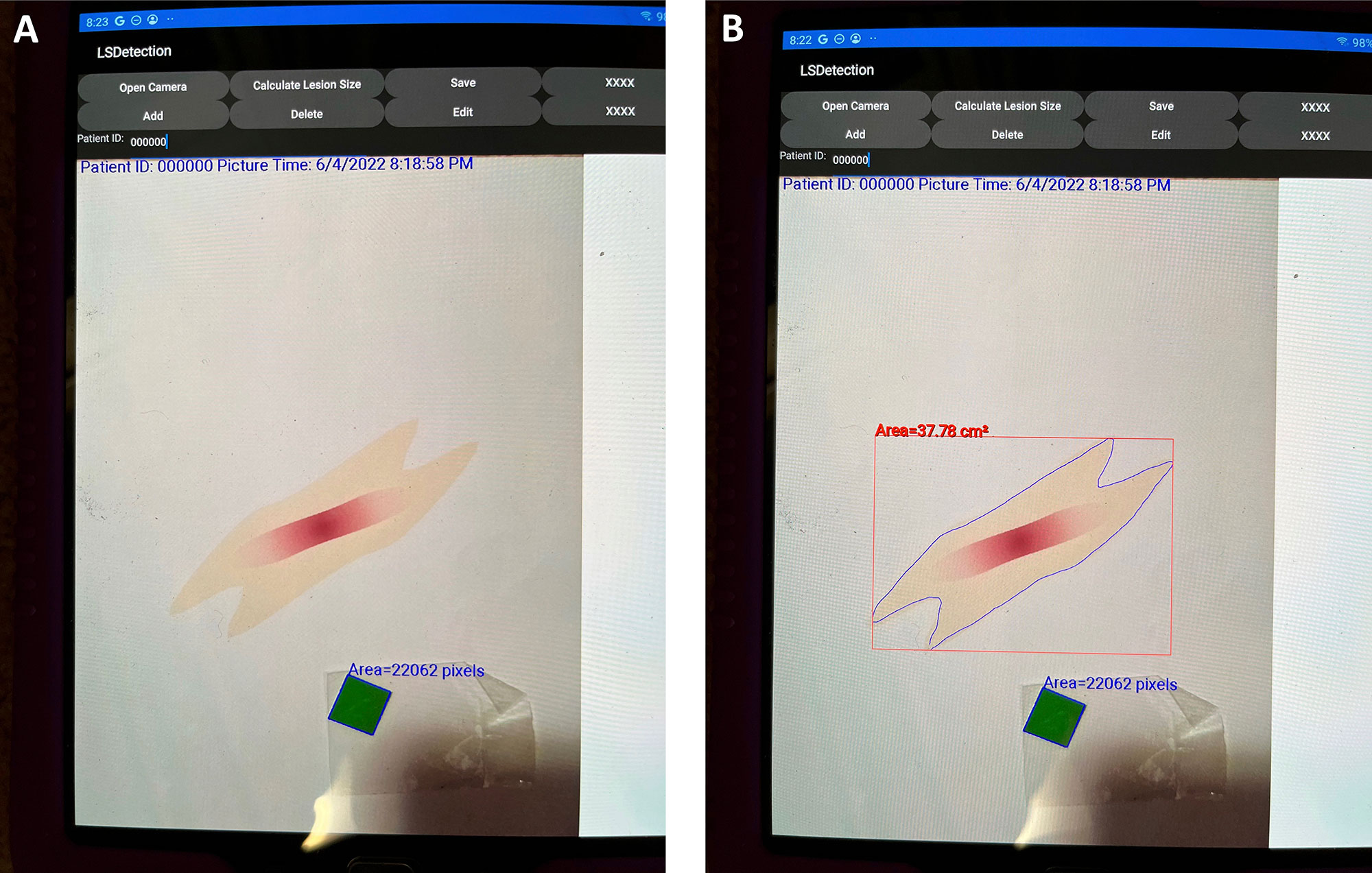
Results: The app layout is shown in Figure 1. The time to photograph shape, upload to app, trace shape with stylus, and calculate the area was 24 seconds for the oval, and 36 seconds for the parallelogram and quatrefoil shapes (shown in Table 1). The overall reproducibility of the app was high, with low coefficients of variation (CV). Raters showed some variability, with Rater 1 showing the lowest CV (1.5-3.4%), and Rater 3 the highest (3.4-5.3%)(Table 1). The accuracy of size determination was >90%, with simpler shapes (oval, parallelogram) potentially showing a higher level of accuracy than more complicated shape (quatrefoil) (Table 1).
Conclusion: Our initial tests of our mobile app for LS show it to be easy and quick to use. In tests with 3 raters, accuracy was 92-98%, and high reproducibility of measurement was found. Future plans include evaluating different scanning conditions and the app’s performance on patient lesions in comparison to other skin measures. This app has the potential to improve monitoring of skin disease activity and long-term outcome in jLS. This app may also facilitate conducting treatment studies in LS.
Three raters (1_3) used the app to calculate the size of test shapes of known sizes. The number of repeat tests done in a given run, mean surface area calculated by the app, and true size of the shape are shown. The coefficient of variation was calculated by dividing the standard deviation of the measurements by the mean area of the shape. and mean accuracy for each run are shown. Abbreviations: cm2: square centimeters; No.: number; SD: standard deviation
A. Shows app with uploaded shape (parallelogram) and green square standard. The app automatically calculates the number of pixels in the standard.
B. After delineating the boundary of the shape with the stylus, the app can calculate the surface area of the shape. The calculated shape is outlined by the red rectangle.
To cite this abstract in AMA style:
Li S, Li X, Lozy T, Chen J. Developing a Mobile App to Facilitate Disease Activity Assessment in Localized Scleroderma [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/developing-a-mobile-app-to-facilitate-disease-activity-assessment-in-localized-scleroderma/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/developing-a-mobile-app-to-facilitate-disease-activity-assessment-in-localized-scleroderma/