Session Information
Session Type: Abstract Session
Session Time: 11:30AM-11:45AM
Background/Purpose: Total cholesterol (TC) and low-density lipoprotein (LDL) are critical measures of atherosclerotic cardiovascular disease (ASCVD) risk. However, the lipid paradox in RA, whereby systemic inflammation reduces lipid levels despite an increased risk of ASCVD, renders TC and LDL unreliable. To account for the lipid paradox, we derived and externally validated inflammation adjusted lipid measures in RA and tested the hypothesis that inflammation adjusted lipids would be more strongly associated with incident ASCVD than unadjusted lipid levels.
Methods: Derivation occurred using baseline data in CERTAIN, a prospective cohort of active RA patients initiating or switching a biologic DMARD. Participants with high sensitivity C-reactive protein (hsCRP) and serum lipids available were included. Statin, JAK inhibitor, and IL-6 inhibitor use were exclusion criteria. Linear spline regression with a single knot was executed using log-transformed hsCRP as a predictor of lipid levels, adjusting for age, sex, smoking status, BMI, diabetes, hypothyroidism, and DMARD use. External validation was conducted in the Veterans Affairs RA registry, a multicenter, prospective RA cohort, with identification of ASCVD events (myocardial infarction, stroke) in linked VA, Medicare, and National Death Index data. The magnitude of the estimated β for hsCRP was used as a correction factor for baseline TC and LDL levels in VARA (TCadj [or LDLadj] = Unadjusted TC [or LDL] + β*log-hsCRP). The association between hsCRP and lipid levels, before and after inflammation adjustment, was illustrated with Lowess smoothing. The association of unadjusted lipids, TCadj and LDLadj values (categorical and dichotomous cutoffs) with incident ASCVD were compared using multivariable Cox regression.
Results: Among 1,663 patients in CERTAIN (mean age 54.4, 81.2% female, mean hsCRP 10.7 mg/L), an inverse U-shaped relationship was observed between hsCRP and unadjusted lipids. A linear spline with a knot at log-hsCRP = 0 yielded the best model fit, leading to a correction factor of 11.4 (TC) and 5.8 (LDL) for each log unit increase in hsCRP above 0 (Figure 1). Adjusting lipid levels for log-hsCRP >0 among 2,106 VARA registry participants (mean age 61.7, 86.4% male, mean hsCRP 11.1 mg/L) corrected the U-shaped relationship (Figure 2) and recategorized a greater proportion of participants into higher TCadj and LDLadj categories (Table 2). Over 17,701 person-years, we observed 371 incident ASCVD events. The associations between categorical and dichotomous TCadj (range aHR 1.35-1.80) and LDLadj (range aHR 1.22-1.56) cutoffs with ASCVD events were stronger than for unadjusted lipids (Table 2).
Conclusion: Compared to unadjusted lipids, inflammation-adjusted TC and LDL identified a larger number of RA patients with high-risk lipid levels and strengthened the association between serum lipids and ASCVD risk. This approach may identify patients with a statin indication who are otherwise missed using current guidelines. Though a critical first step in overcoming challenges to care posed by the lipid paradox, additional study is required to integrate these results into risk calculators to optimize CVD prevention in RA.
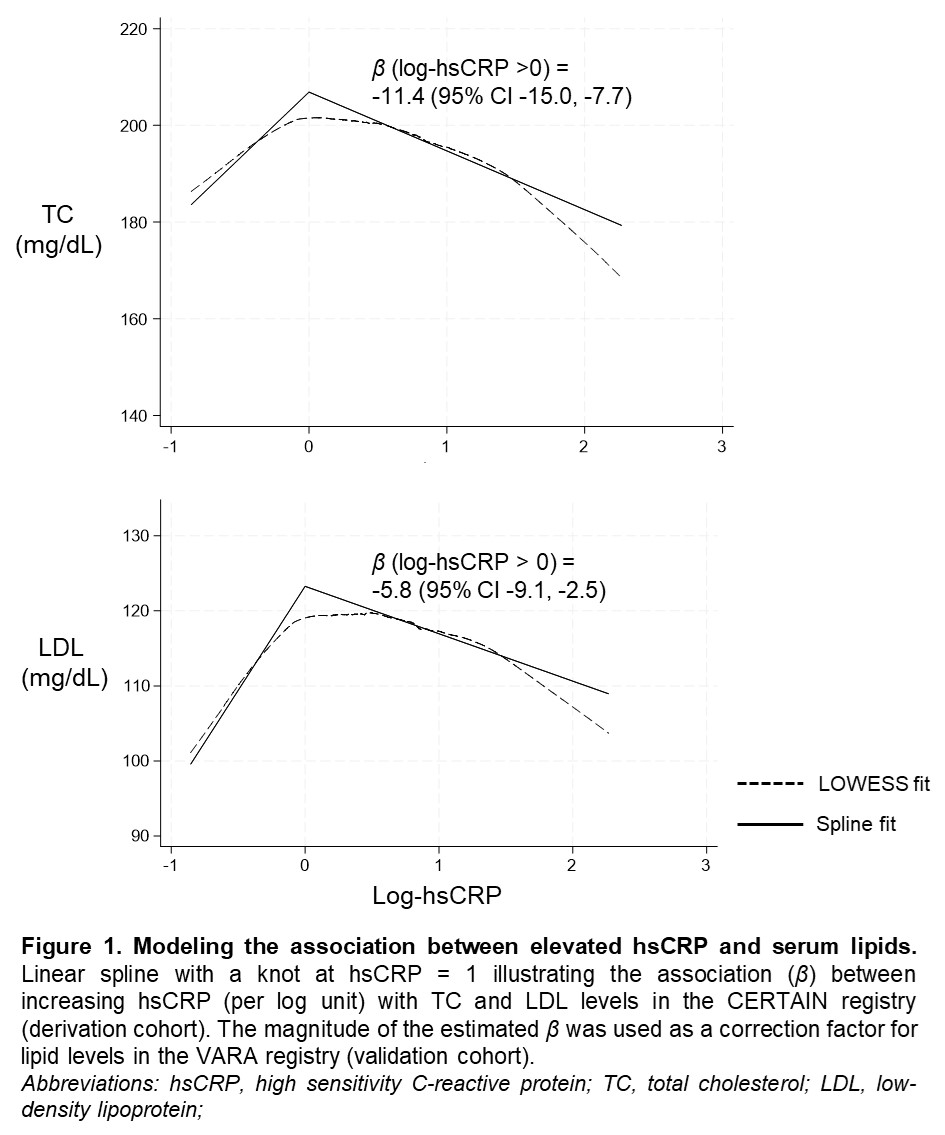 Figure 1. Modeling the association between elevated hsCRP and serum lipids.
Figure 1. Modeling the association between elevated hsCRP and serum lipids.
.jpg) Figure 2. Association of hsCRP with serum lipids before and after inflammation adjustment.
Figure 2. Association of hsCRP with serum lipids before and after inflammation adjustment.
.jpg) Table 1. Validation of Inflammation Adjusted Lipid Measures with Incident Cardiovascular Events
Table 1. Validation of Inflammation Adjusted Lipid Measures with Incident Cardiovascular Events
To cite this abstract in AMA style:
Johnson T, Reed G, Kremer J, Pappas D, Roul P, Cannon G, Kerr G, Reimold A, Liao K, George M, Giles J, Charles-Schoeman C, Baker J, Mikuls T, England B. Derivation and Validation of Inflammation-Adjusted Lipid Measures to Improve Cardiovascular Risk Prediction in Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/derivation-and-validation-of-inflammation-adjusted-lipid-measures-to-improve-cardiovascular-risk-prediction-in-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/derivation-and-validation-of-inflammation-adjusted-lipid-measures-to-improve-cardiovascular-risk-prediction-in-rheumatoid-arthritis/
