Background/Purpose: Inclusion body myositis (IBM), a relentlessly progressive autoimmune skeletal muscle disease, has no effective available pharmacological therapy. A prominent feature of IBM on microscopy is highly differentiated effector CD8+ cytotoxic T (Tc) cells invading non-necrotic myofibers1. These Tc cells are known to be relatively resistant to apoptosis and express markers including killer cell lectin‑like receptor G1 (KLRG1)2. ABC008, a first-in-class humanized, afucosylated monoclonal antibody (mAb) specific for KLRG1, selectively depletes these highly differentiated Tc cells while sparing other blood cell populations, e.g., naïve, central memory, and regulatory T cells. ABC008 has been designed to treat diseases mediated by these Tc cells, including IBM and T-large granular lymphocytic leukemia (T-LGLL). IBM as well as rheumatoid arthritis overlap clinically with T-LGLL and share similar expansions of blood large granular lymphocytes (LGLs), which also express KLRG1+. We report here our preliminary data from our ongoing trial of ABC008 in IBM (NCT04659031).
Methods: In this ongoing first-in-human, open-label, single ascending dose trial with 3+3 design evaluating ABC008 administered subcutaneously (SC), eligible participants must have clinicopathologically defined, clinically defined, or probable IBM according to the European Neuromuscular Centre 2011 research diagnostic criteria2 and an IBM Functional Rating Scale score of ≤38. Four dose cohorts are planned: ABC008 0.1, 0.5, 2.0, and 5.0 mg/kg. Pharmacodynamic, pharmacokinetic (PK), safety, and disease severity assessments are performed pre‑dose (Day 0) and during the 6‑month follow-up period.
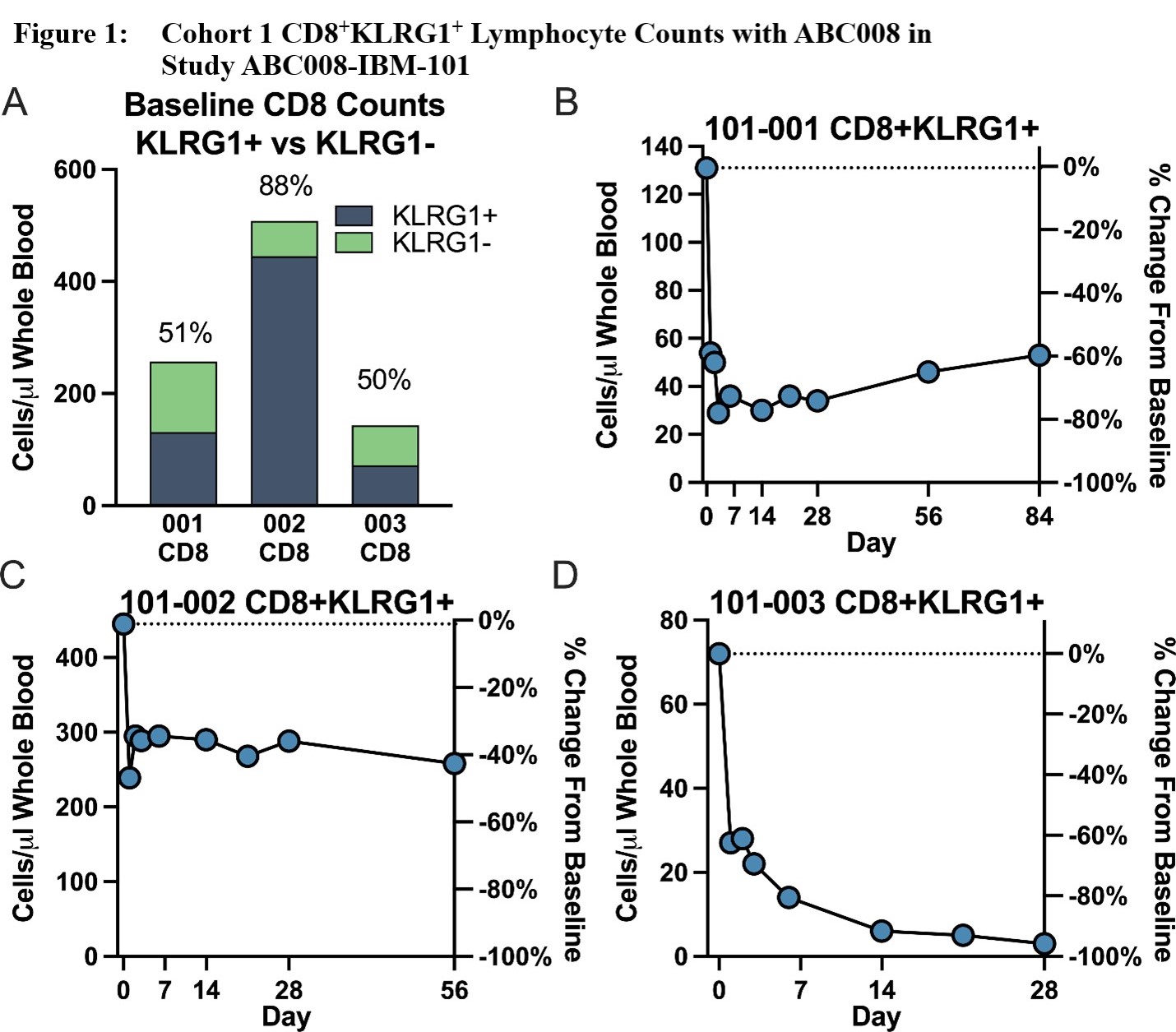
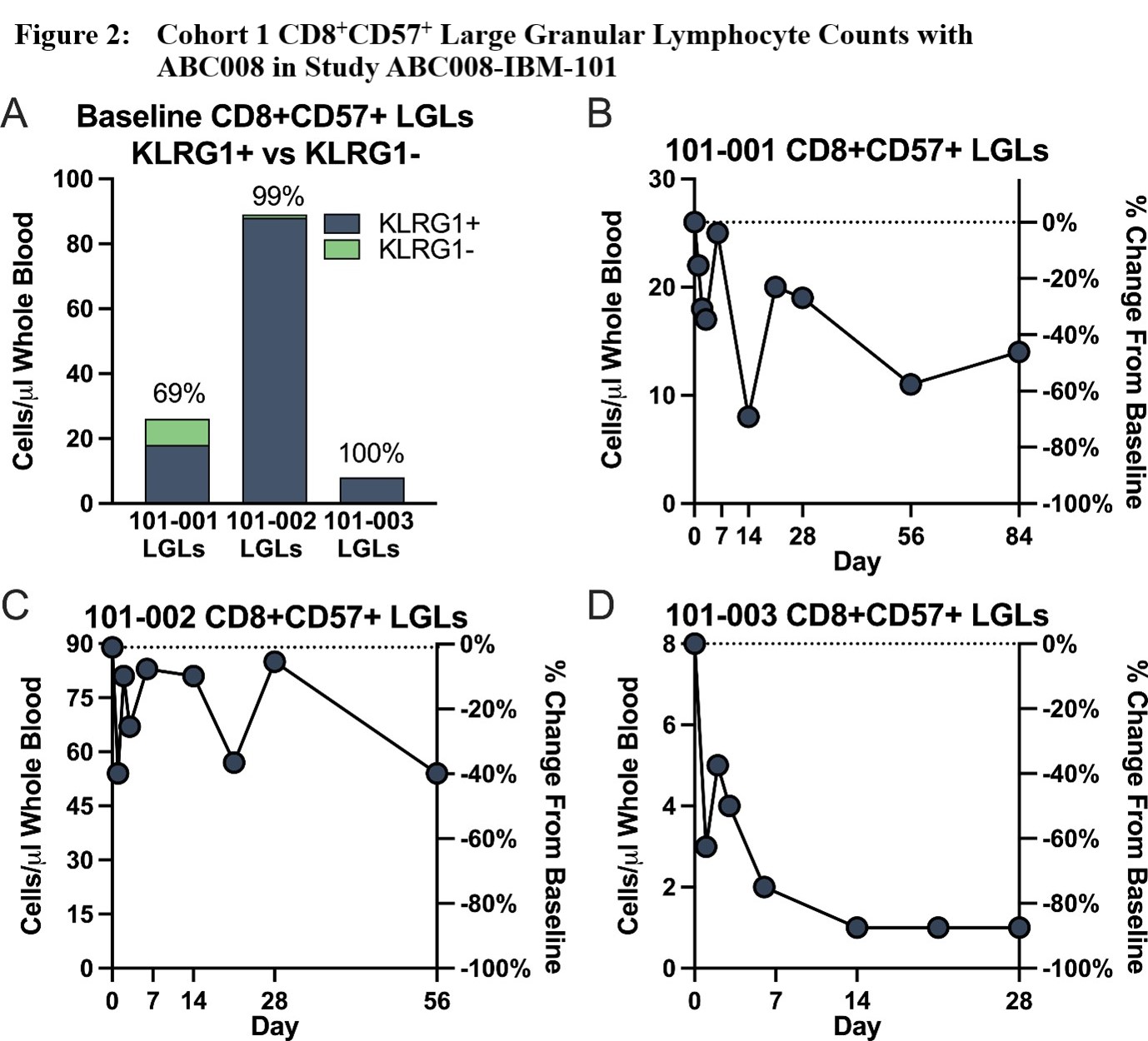
Results: Three Caucasian male participants, mean age ± standard deviation (SD) = 64 ± 11.4 years, mean IBM disease duration ± SD = 9.7 ± 5.91 years, and mean baseline IBMFRS ± SD = 30 ± 5, were each dosed with ABC008 at 0.1 mg/kg SC x 1 and completed ≥28 days of follow-up. Maximum depletion of CD8+KLRG1+ cells (Figure 1) through Day 28 ranged from 46‑96% of baseline, with depletion evident from Day 2 onward and largely maintained through at least Day 28 (mean depletion 68%). Other hematologic parameters generally remained stable including T regulatory cells. CD8+ LGLs, because they are mostly KLRG1+, were also depleted (Figure 2). Preliminary PK show that ABC008 displays a long absorption phase following SC administration and slow clearance properties typical of mAb therapies. No moderate, severe, or serious adverse events were reported.
Conclusion: In study participants with IBM, a single SC dose of 0.1 mg/kg of ABC008 resulted in the depletion of CD8+KLRG1+ cells with no apparent safety concerns. Higher dose cohorts are planned. Based on these results, a study evaluating ABC008 for the treatment of T-LGLL is also intended.
1. Engel AG, et al. Ann Neurol. 1984;16:209-15.
2. Greenberg SA, et al. Brain. 2016;139:1348-60.
3. Rose MR, et al. Neuromuscul Disord. 2013;23:1044–55.
To cite this abstract in AMA style:
Goel N, Soler-Ferran D, Coutreau M, Escobar J, Courtemanche K, Needham M, Greenberg S. Depletion of KLRG1+ T Cells in a First-in-human Clinical Trial of ABC008 in Inclusion Body Myositis (IBM) [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/depletion-of-klrg1-t-cells-in-a-first-in-human-clinical-trial-of-abc008-in-inclusion-body-myositis-ibm/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/depletion-of-klrg1-t-cells-in-a-first-in-human-clinical-trial-of-abc008-in-inclusion-body-myositis-ibm/