Session Information
Date: Saturday, November 16, 2024
Title: Abstracts: T Cell Biology & Targets in Autoimmune & Inflammatory Disease
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Systemic lupus erythematosus (SLE) is a leading cause of death in young females, and 70-85% of patients experience skin disease (cutaneous LE, CLE). The mechanisms of CLE pathogenesis remain unknown, and current treatments rely on broad immunosuppressive agents. Mucosal-associated invariant T (MAIT) cells, innate-like T cells that respond to microbial-derived antigens, have tissue repair capacity at homeostasis but are reduced in CLE skin. We hypothesize that restoring MAIT cell pools in non-lesional CLE-like skin will attenuate disease.
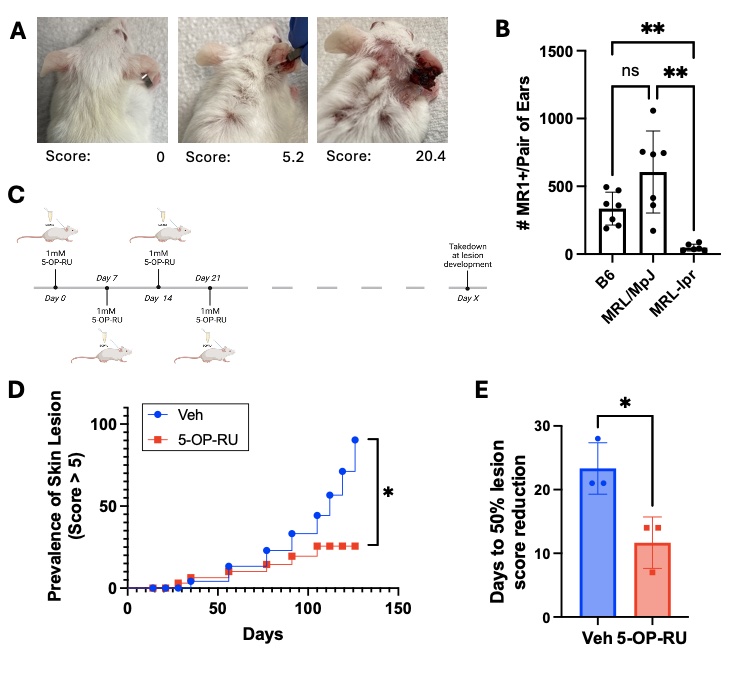
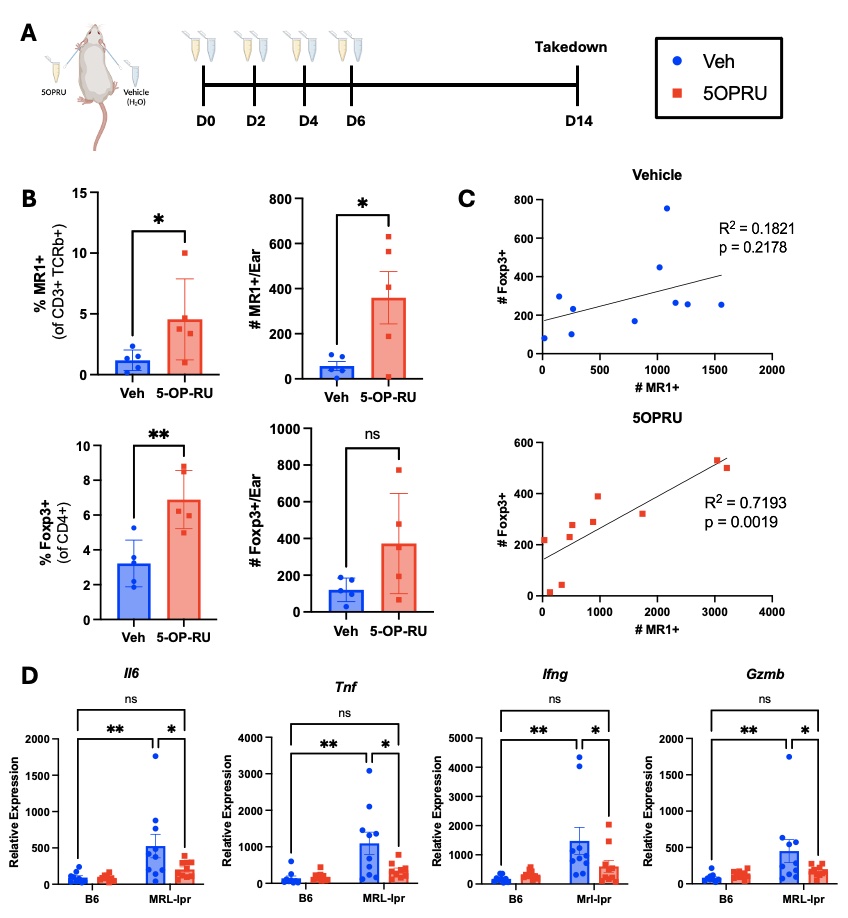
Methods: To test if expanding MAIT cells slows CLE development, female MRL-lpr mice were treated topically with the MAIT cell antigen 5-OP-RU (1mM) weekly starting at 8 weeks of age, and skin lesions were photographically documented and quantified by extent of erythema, scaling, thickness, and alopecia (Fig. 1A). To assess how MAIT cell expansion affects skin immune and inflammatory environment, 8-week-old female MRL-lpr mice were treated with 5-OP-RU topically every 48 hours for 4 total applications (Fig. 2). Skin immune and inflammatory environment was assessed by flow cytometry and qPCR.
Results: Analogous to the observations in the skin of lupus patients, MRL-lpr mice have fewer MAIT cells in non-lesional skin, compared to the control MRL/MpJ and B6 strains (Fig. 1B). Weekly topical application of the MAIT cell antigen 5-OP-RU, beginning prior to lesion development (Fig. 1C), reduced the prevalence of severe skin lesions compared to vehicle treated controls (Fig. 1D). Of the mice that did develop lesions, those that received 5-OP-RU displayed accelerated lesion healing (Fig. 1E). On average, 5-OP-RU-treated mice exhibited 50% reduction in lesion score 7 days faster than vehicle treated controls. 5-OP-RU topical application effectively expanded MAIT cells in Mrl-lpr skin (Fig. 2A). Surprisingly, MAIT expansion was accompanied by a concurrent increase in regulatory T cells (Treg) in the skin (Fig. 2B), and Treg numbers positively correlated with MAIT cell numbers (Fig. 2C). Moreover, MAIT cell expansion with 5-OP-RU reduced the high baseline expression of inflammatory (Il-6, Tnf) and cytotoxic (Ifng, Gzmb) genes in MRL-lpr skin, to the low levels of healthy B6 skin (Fig. 2D).
Conclusion: Topical application of the MAIT cell antigen 5-OP-RU slows development and progression of CLE-like lesions, suggesting a protective role for these cells in the skin. The concurrent expansion of Treg supports a potential immunosuppressive mechanism by which MAIT cells downregulate the expression of inflammatory and cytotoxic mediators in MRL-lpr skin associated with lesion development.
To cite this abstract in AMA style:
Crossland G, Mendyka L, Constantinides M, Skopelja-Gardner S. Defining a Protective Role for Mucosal-associated Invariant T (MAIT) Cells in Spontaneous Cutaneous Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/defining-a-protective-role-for-mucosal-associated-invariant-t-mait-cells-in-spontaneous-cutaneous-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/defining-a-protective-role-for-mucosal-associated-invariant-t-mait-cells-in-spontaneous-cutaneous-lupus-erythematosus/