Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Maternal anti-SSA/Ro52/60kD autoantibodies are necessary for the development of fetal atrioventricular block (AVB) but titers alone are not sufficient to predict the likelihood of AVB. Thus, optimal surveillance for early detection and treatment, the design of clinical trials for prophylaxis, and a more complete understanding of pathogenesis remain elusive. Despite increasing the predictive value for fetal AVB by excluding pregnant subjects with low titer anti-Ro antibodies, the 60% of individuals with titers above the risk threshold with no prior affected offspring still face only a 4% risk of developing fetal AVB. In search of additional biomarkers for more accurate risk assessment, this study sought to identify novel autoantibodies associated with fetal AVB in high titer anti-Ro pregnant subjects.
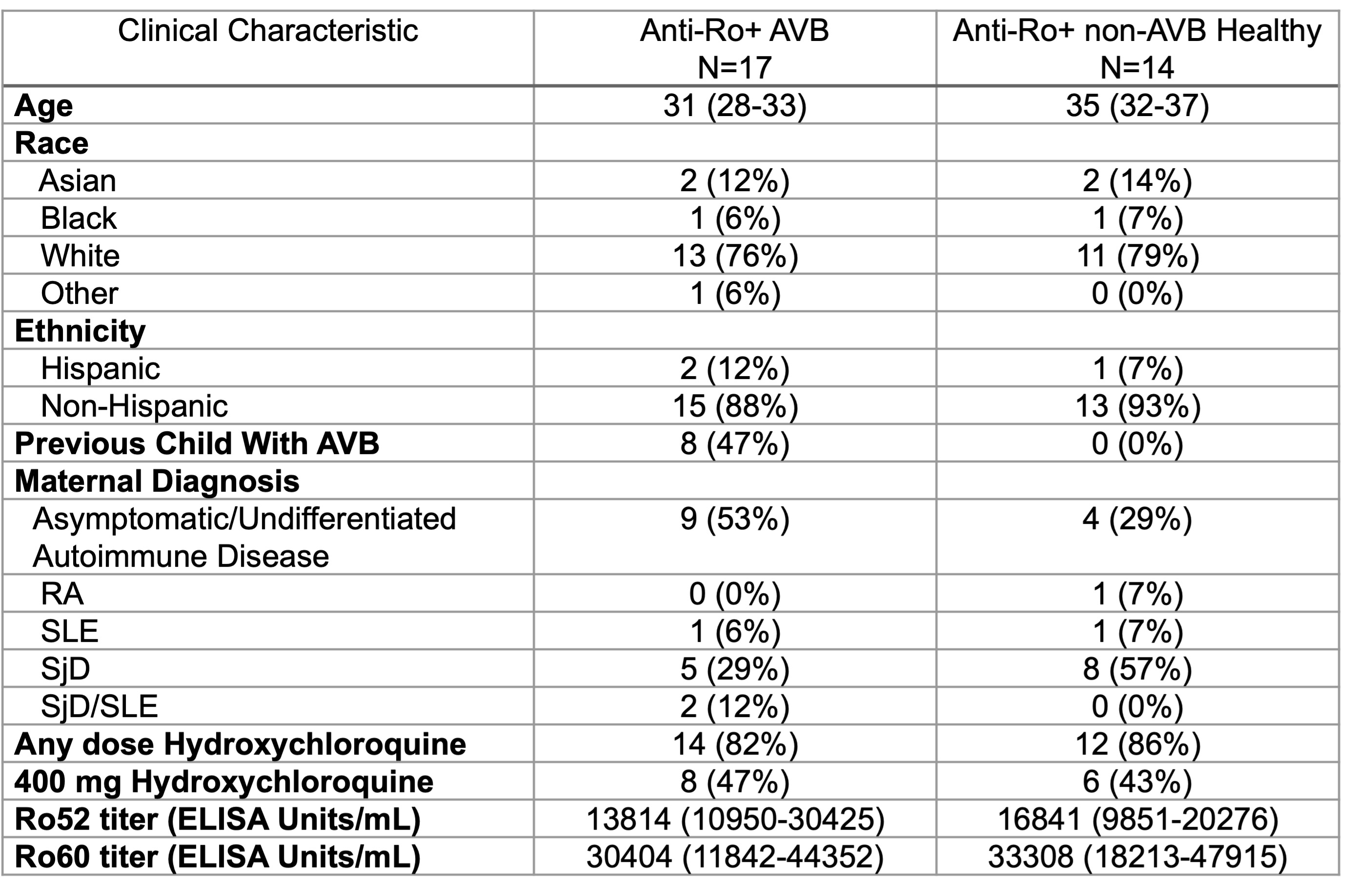
Methods: Sera from 17 AVB affected pregnancies (15 collected prospectively prior to week 20 during enrollment in the STOP BLOQ or PATCH trial and before the development of AVB and 2 collected at delivery) were matched to 14 high titer anti-Ro exposed healthy pregnancies (all collected prior to week 20) based on race/ethnicity, maternal rheumatologic diagnosis, anti-Ro52 and 60 titers (performed by ELISA in our research lab), and hydroxychloroquine dose to eliminate potential factors conferring a change in risk. Five samples from anti-Ro negative healthy pregnant women were also included. IgG antibodies against native human proteins were assessed by the HuProt array (CDI Laboratories). Proteins binding directly to secondary antibodies without serum were excluded and raw data for the remaining 23058 proteins were quantile normalized. Candidate proteins met the following criteria: p< 0.05 by t-test on log transformed data and fold change >1.5 comparing AVB and anti-Ro+ non-AVB pregnancies and at least four ( >20%) AVB samples with values >3 standard deviations above the mean of the anti-Ro- healthy controls.
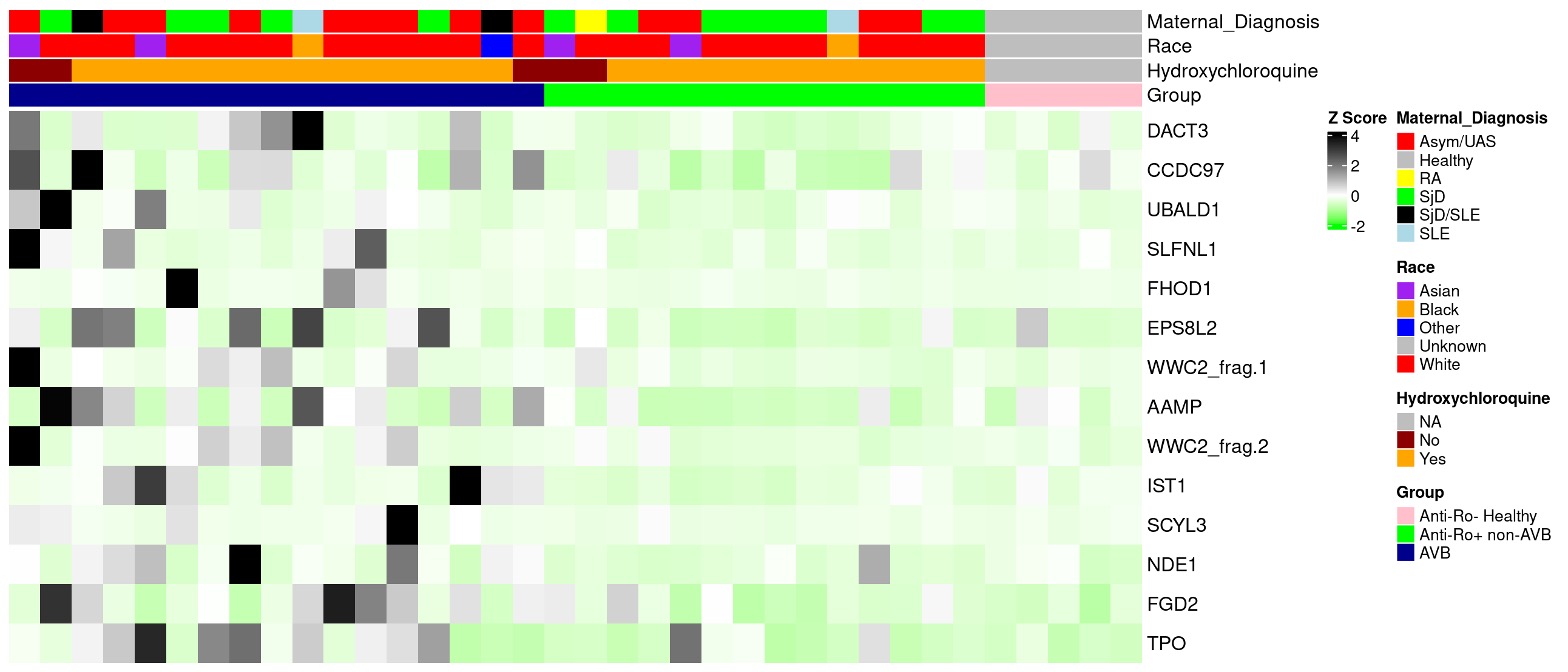
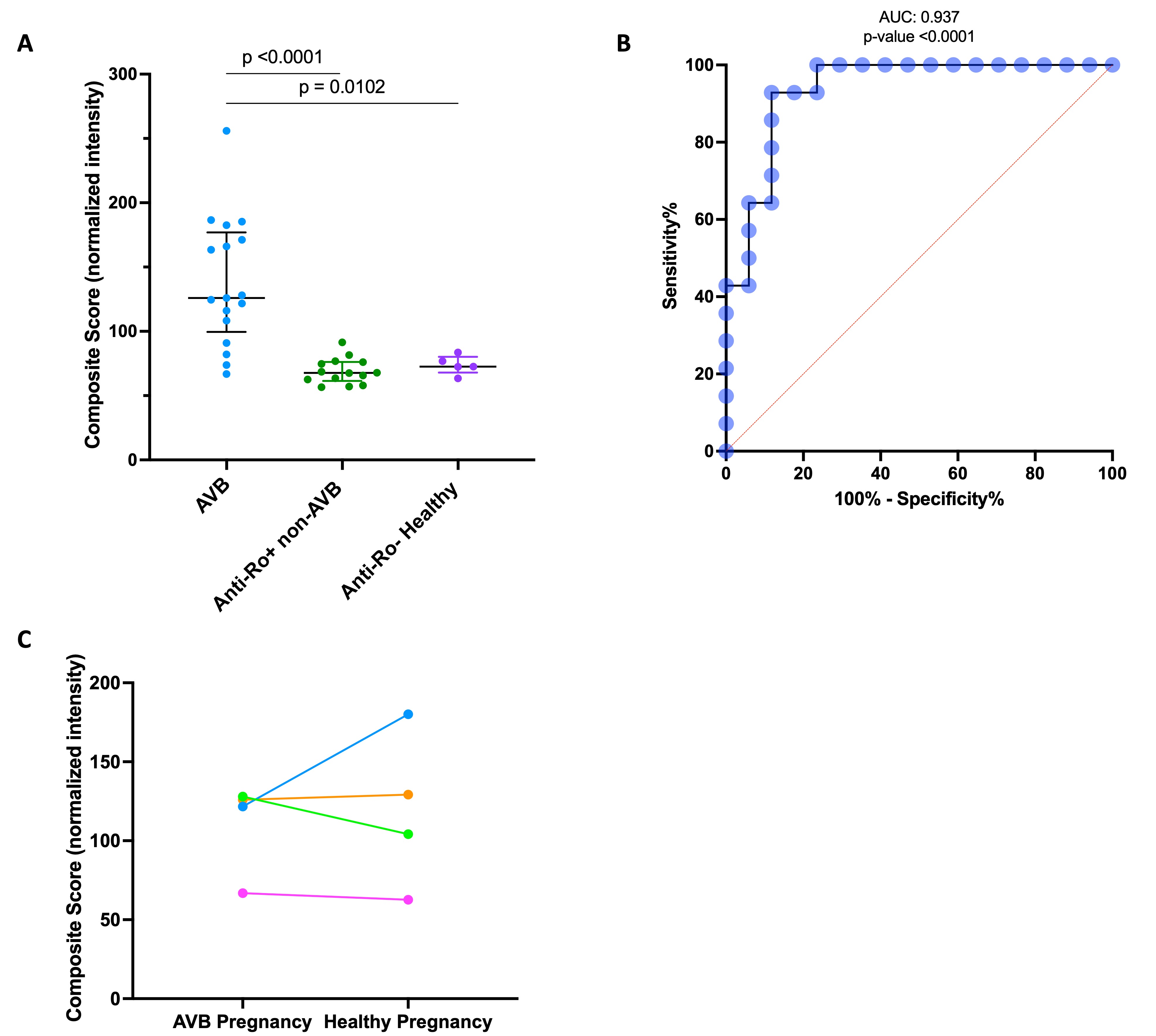
Results: The 17 AVB affected pregnancies and 14 anti-Ro+ non-AVB pregnancies were well matched on the selected clinical variables (Table 1). As a validation, the levels of anti-Ro52 and Ro60 measured by ELISA significantly correlated with those on the autoantibody array. As expected, the highest array intensities corresponded to Ro52 and 60. The discovery array revealed antibodies against 13 unique proteins that were higher in AVB vs anti-Ro+ non-AVB pregnancies (Fig 1). A composite score was calculated for each subject by averaging the normalized values of the candidate antigens (Fig 2A). This score discriminated between AVB and anti-Ro+ non-AVB pregnancies with an AUC of 0.937 (p-value < 0.0001) (Fig 2B). Samples from 4 of the 17 AVB pregnant subjects during an unaffected healthy pregnancy were also evaluated. The composite score remained strikingly similar between these pregnancies, suggesting that the candidate antibodies may serve as stable biomarkers of enhanced maternal risk but are not fully predictive of disease (Fig 2C).
Conclusion: This high-throughput evaluation provides insights into novel autoantibodies associated with the development of fetal AVB in anti-Ro exposed pregnancies and could be used to generate predictive biomarkers that identify pregnancies at highest risk.
To cite this abstract in AMA style:
Carlucci P, Clancy R, Masson M, Phoon C, Roman A, Izmirly P, Saxena A, Belmont H, Penfield C, Lee Y, Nusbaum J, Rubenstein A, Acherman R, Sinkovskaya E, Abuhamad A, Bermudez-Wagner K, Makhoul M, Satou G, Hogan W, Pinto N, Moon-Grady A, Howley L, Donofrio M, Krishnan A, Phillips J, Levasseur S, Geiger M, Paul E, Owens S, Cumbermack K, Matta J, Joffe G, Lindblade C, Weiner C, Haxel C, Kohari K, Copel J, Strainic J, Doan T, Sheth S, Killen S, Tacy T, Kaplinski M, Fraser N, Ruggles K, Cuneo B, Buyon J. Deep Serologic Profiling Identifies Novel Autoantibodies Associated with Fetal Atrioventricular Block [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/deep-serologic-profiling-identifies-novel-autoantibodies-associated-with-fetal-atrioventricular-block/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/deep-serologic-profiling-identifies-novel-autoantibodies-associated-with-fetal-atrioventricular-block/