Session Information
Date: Sunday, November 13, 2022
Title: Abstracts: RA – Treatment I: Switching or Discontinuation of Therapies
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Rheumatoid arthritis (RA) patients with inadequate response to methotrexate often experience trial-and-error treatment selection due to a lack of guidance from clinical guidelines or validated biomarkers that can predict response to biologic or targeted-synthetic DMARD (b/tsDMARD) therapy. This approach leads to frequent therapy discontinuation and switching, resulting in greater healthcare costs without necessarily improving patient outcomes. A blood-based molecular signature response classifier (MSRC) has been validated to predict RA patients who will be non-responders to tumor necrosis factor-alpha inhibitors (TNFi), a commonly prescribed biologic treatment for RA. This study examined the impact of treatment discontinuation and switching of b/tsDMARD therapy according to treatment selection alignment with results of the MSRC.
Methods: Analyses were conducted in the Study to Accelerate Information of Molecular Signatures (AIMS), a longitudinal, prospective clinical study of RA patients across a network of rheumatology practices in the US. Patients initiating a b/tsDMARD after MSRC testing with a 6-month follow-up visit were included. Patients were classified according to whether they initiated a TNFi or an alternative mechanism of action (altMOA), and within the TNFi-treated group, whether the MSRC detected a signature of predicted non-response (Unaligned-TNFi) or not (Aligned-TNFi). Discontinuation was defined as the termination of a drug prescribed at treatment decision, and a switch was defined by the initiation of a new b/tsDMARD. Kaplan-Meier curves were used to estimate the time to discontinuation or switch. Cox proportional hazard models were used to adjust for age, sex, and baseline clinical disease activity index (CDAI), tender joint count, and TNFi-naïve status.
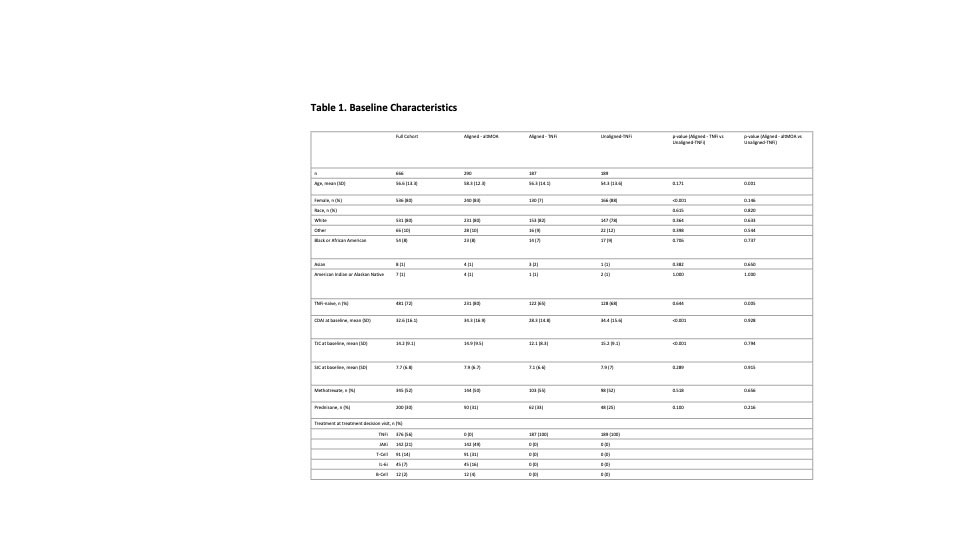
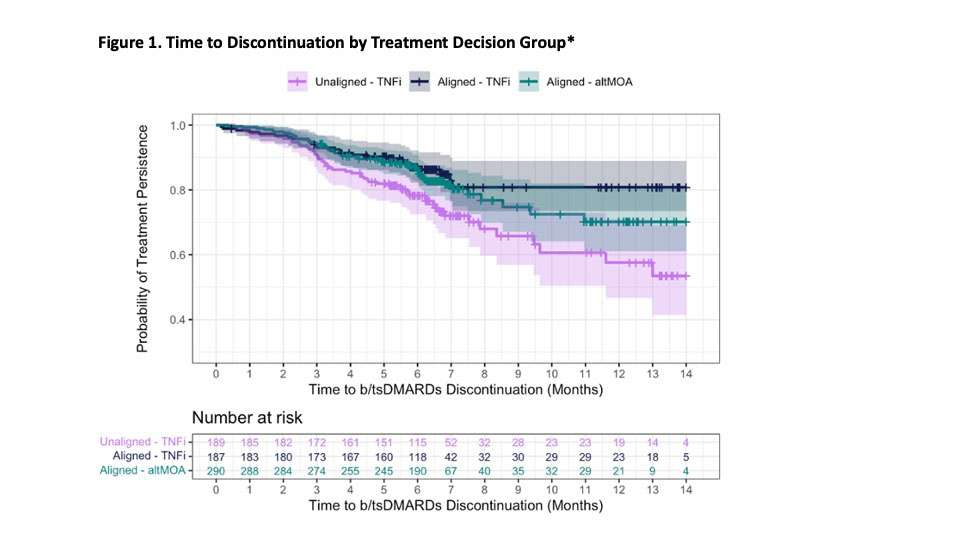
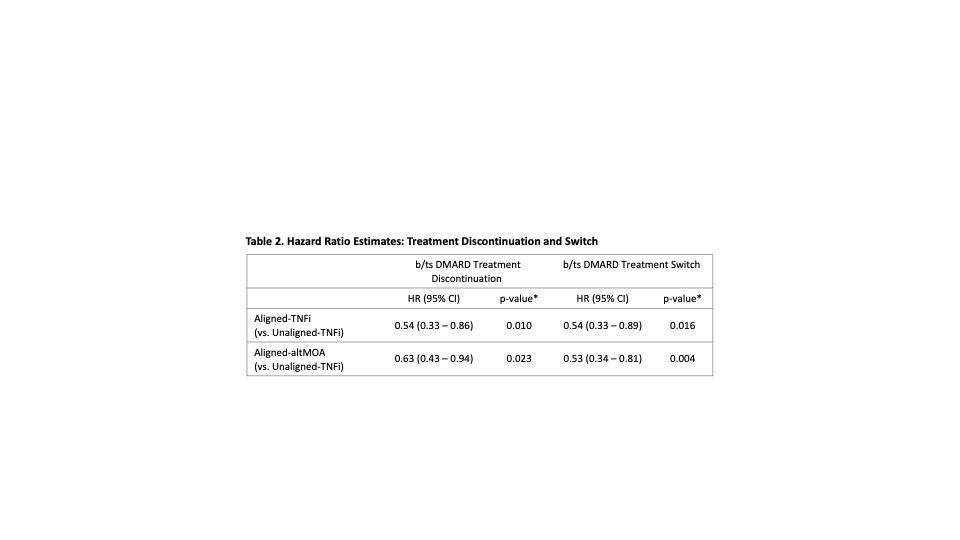
Results: A total of 666 patients were included: the mean age was 56.6 (SD=13.3) years, 80% were female, baseline CDAI was 32.6 (SD=16.1), 72% were TNFi-naïve (Table 1). Most patients (72%) had a treatment decision aligned with the MSRC result, of which 39% were prescribed a TNFi. The Unaligned-TNFi group, which consisted of patients with a signature of predicted non-response to TNFi yet were placed on a TNFi anyway, had a higher proportion of discontinuation compared to the Aligned group (Fig. 1). A similar trend appeared in the proportion of patients who switched drug therapies. Adjusted analyses showed the Aligned group was less likely to discontinue treatment (Table 2. Aligned-TNFi HR=0.54, 95% CI: 0.33 – 0.86; Aligned-altMOA HR=0.63, 95% CI: 0.43 – 0.94) and less likely to switch therapy than the Unaligned-TNFi group (Table 2. Aligned-TNFi HR=0.54, 95%CI: 0.33 – 0.89; Aligned-altMOA HR=0.53, 95%CI: 0.34 – 0.81).
Conclusion: When rheumatologists selected a b/tsDMARD therapy aligned with a predictive MSRC, patients experienced less therapy discontinuation and switching than those who were treated with a TNFi when the MSRC predicted future non-response. This study further demonstrates the value of precision medicine in RA in which a treatment paradigm informed by a validated biomarker test, such as the MSRC, is used to prescribe medications.
To cite this abstract in AMA style:
Curtis J, Rusli E, Zhang L, Le-Short C, Arnaud A, Withers J, Asgarian S. Decreased Discontinuation and Switching of B/tsDMARD Therapy in RA Patients When Treatment Is Aligned with a Molecular Signature Response Classifier: An Analysis from the Study to Accelerate Information of Molecular Signatures (AIMS) [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/decreased-discontinuation-and-switching-of-b-tsdmard-therapy-in-ra-patients-when-treatment-is-aligned-with-a-molecular-signature-response-classifier-an-analysis-from-the-study-to-accelerate-informati/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/decreased-discontinuation-and-switching-of-b-tsdmard-therapy-in-ra-patients-when-treatment-is-aligned-with-a-molecular-signature-response-classifier-an-analysis-from-the-study-to-accelerate-informati/