Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Nephritis is one of the most severe manifestations of lupus, affecting 40-70% of patients. Though immune-targeted therapies have improved, a significant number of patients experience renal damage and even progression to end-stage renal disease (ESRD). Recently, sodium and glucose cotransporter 2 inhibitors (SGLT2i) have proven efficacious in preventing adverse renal outcomes in patients with a variety of chronic diseases. The mechanisms of protection by SGLT2i include their capacity to modulate hypoxia and fibrosis. Other literature supports the role of local hypoxia in the lupus nephritis (LN) kidney in driving pathogenic immune cell function, including in CD8 T cells. Here, we hypothesized that by alleviating hypoxia, SGLT2i will modulate local inflammation and fibrosis in LN.
Methods: Ten weeks old MRL/lpr female mice were treated daily with dapagliflozin at 10 mg/kg or vehicle by gavage for 11 weeks. Sera was collected to measure autoantibodies and creatinine by ELISA. Immune cells and Sodium and Glucose Co-Transporter 2 (SGLT2) expression were quantitated in the spleen, lymph nodes (LNs) and kidneys by flow cytometry and immunofluorescence. Inflammation, fibrosis, and infiltration by CD8 T cells or regulatory T cells were assessed in the kidneys of MRL/lpr mice at baseline and 11 weeks after starting SGLT2i therapy.
Results: Consistent with a slow induction of SGLT2i therapeutic effect in humans and despite the efficient targeting of SGLT2 in MRL/lpr mice, proteinuria was not affected after 11 weeks of daily SGLT2i administration. Unexpectedly SGLT2i therapy had a remarkable impact on the formation of germinal centers (GCs) (vehicle vs. dapagliflozin: *, p = 0.0205) and the production of autoreactive plasma cells in the spleen (vehicle vs. dapagliflozin: **, p = 0.009). In line with the induction of regulatory T cells by SGLT2i in diabetic kidney disease, T follicular helper cells with a regulatory phenotype significantly increased in the GCs of mice treated with dapagliflozin (vehicle vs. dapagliflozin: **, p = 0.003). In addition, skin and lymph node inflammation were attenuated by SGLT2i. However, inflammatory cell infiltration in the kidney was not affected by SGLT2i.
Conclusion: Despite modest effects on kidney inflammation, SGLT2i surprisingly modulated splenic autoreactive GCs via significant accumulation of T follicular regulatory T cells, impairing the production of autoreactive plasma cells. One of the potential explanations for the modest kidney effects is the rapid disease progression in MRL/lpr mice and the slow therapeutic induction with SGLT2i. Thus, future studies are planned in NZB/NZW mice treated for longer periods of time and with combined immune suppressive therapy.
Supported by a Lupus Mechanisms and Targets Award from the Lupus Research Alliance
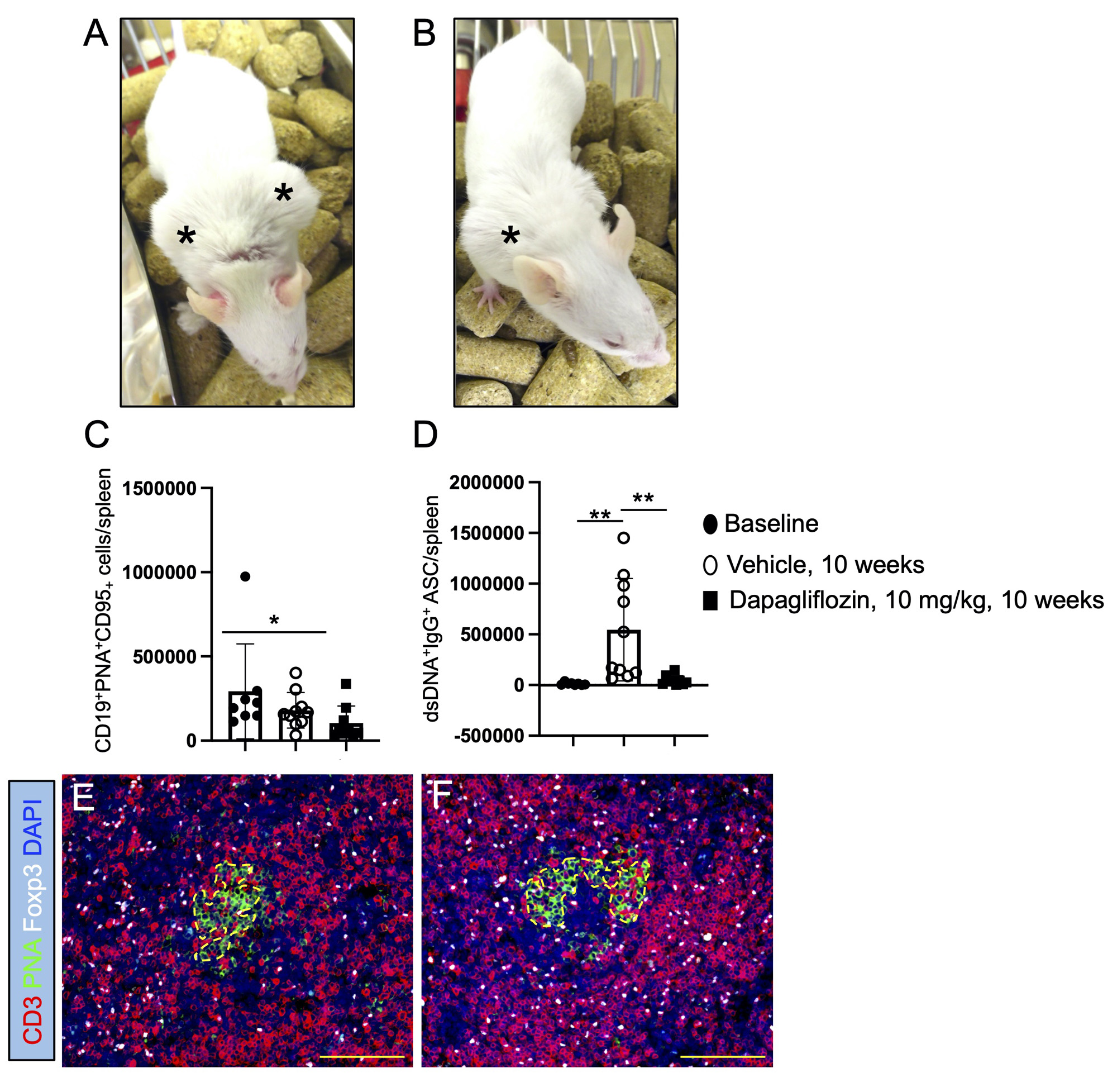
MRL/lpr female mice were daily treated for 10 weeks with dapagliflozin at 10 mg/kg by gavage
or received same volume of vehicle. A) MRL/lpr female mice receiving vehicle show increased
inflammation in peripheral lymph nodes, compared to B) aged matched MRL/lpr mice treated
with dapagliflozin. Asterix point to inflamed peripheral lymph nodes (LNs). C) Germinal center
B cells were decreased in the spleen of MRL/lpr female mice treated with dapagliflozin for 11
weeks. D) Production of autoreactive dsDNA antibody-secreting cells (ASC) in the spleen was
significantly reduced by dapagliflozin therapy. E) T follicular regulatory T cells (TFHregs) were
less numerous in the germinal centers (GCs) and contiguous areas in the LNs of control mice,
compared to F) the significant accumulation of TFHregs in GCs of dapagliflozin-treated mice.
200x magnification pictures. Scale bars = 100 µm. n = 8_10 mice/group. Unpaired t test, Mann
Whitney test: *, p ≤ 0.05, **, p ≤ 0.005.
To cite this abstract in AMA style:
Rangel-Moreno J, Garcia-Hernandez M, O'Connell M, Krenitsky D, Ossa-Echeverri M, Lusco M, Looney J, Anolik J. Dapagliflozin Modulates Inflammation and Germinal Centers in Lupus by Increasing Regulatory T Cells [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/dapagliflozin-modulates-inflammation-and-germinal-centers-in-lupus-by-increasing-regulatory-t-cells/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/dapagliflozin-modulates-inflammation-and-germinal-centers-in-lupus-by-increasing-regulatory-t-cells/