Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: NR4A family regulates T cell tolerance mechanisms, including thymic-negative selection, Treg generation and function, and anergy/exhaustion. Among the three NR4A nuclear receptors, Nur77, the product of Nr4a1 gene, is well-characterized in T and B cells. A recent study revealed that Nur77 could act as a brake during T-cell activation in acute phase of T cell receptor stimulation. Another study elucidated that higher expression of Nr4a1 in CD4+ T cells marked their auto-reactivity and arthritogenicity in experimental arthritis mice. In experimental autoimmune encephalomyelitis, cytosporone B (CsnB), a specific agonist targeting Nr4a1, can enhance the functions of Nur77 and ameliorate disease activity. This study aimed to assess the impact of CsnB on T cells and explore its therapeutic potential in a murine arthritis model.
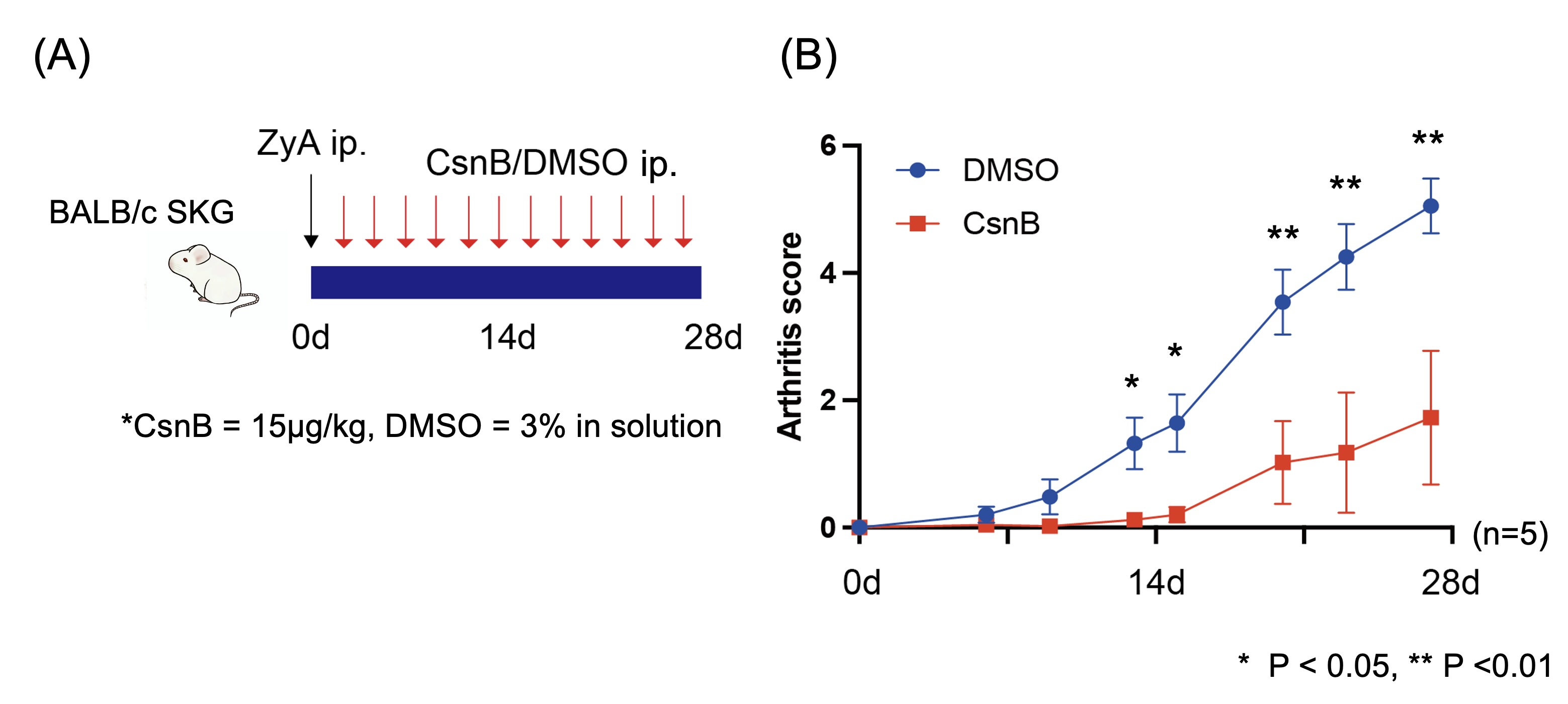
Methods: In vitro, activation markers of CD4+ T cells (CD69, CD25, PD-1) were assessed following anti-CD3/28 stimulation in the presence or absence of CsnB by flow cytometry. The effect of CsnB on in vitro Th17 differentiation was assessed by flow cytometry. During Th17 differentiation experiments, phosphorylation of STAT3 and transcriptome were also assessed. In vivo, arthritis was induced in SKG mice by Zymosan A injection. DMSO (control) or CsnB (15μg/kg) was injected intraperitoneally 3 times/week from the day after Zymosan A injection. The effect of CsnB (compared to DMSO) on arthritis was assessed, and splenocytes and synovial cells were studied by flow cytometry.
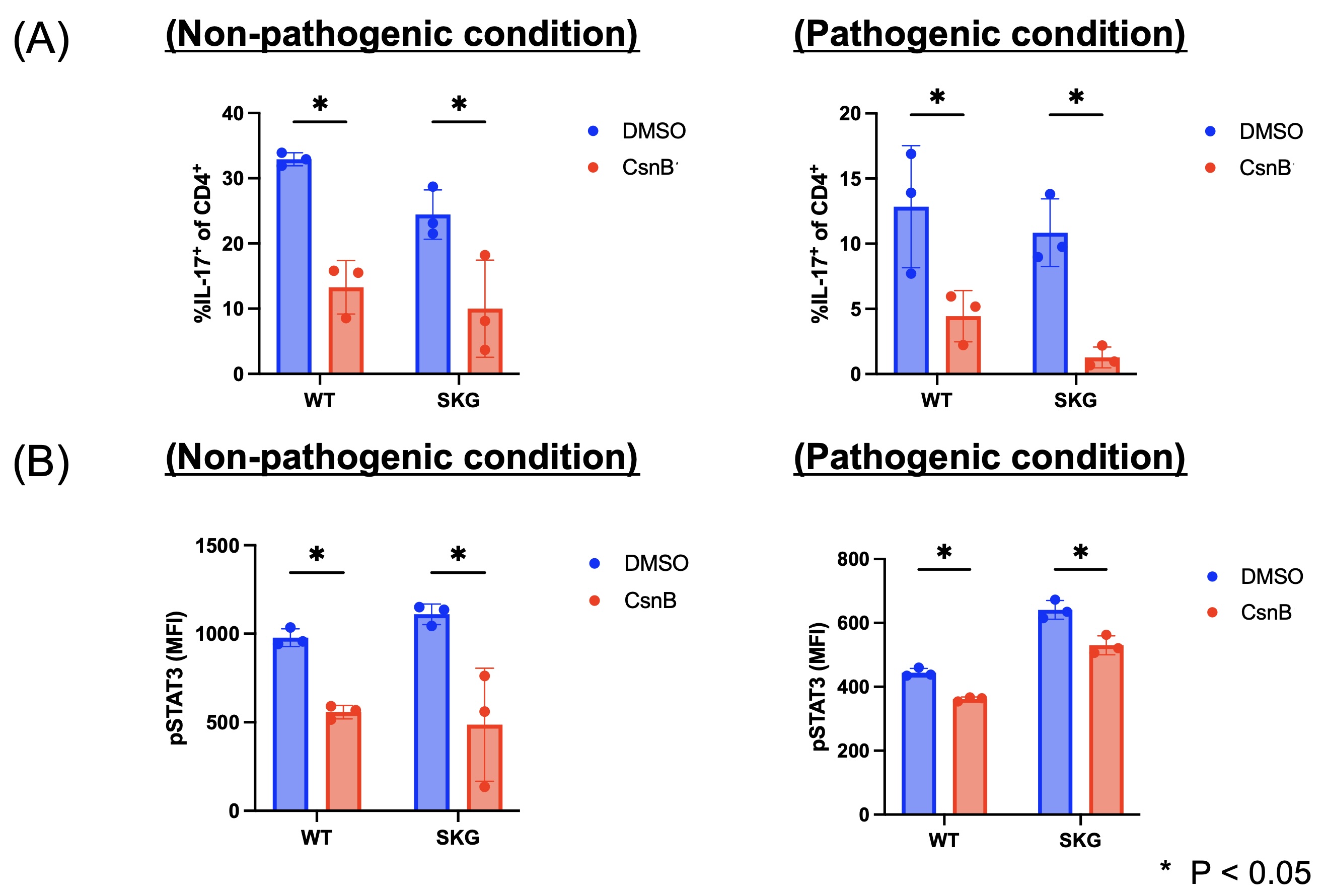
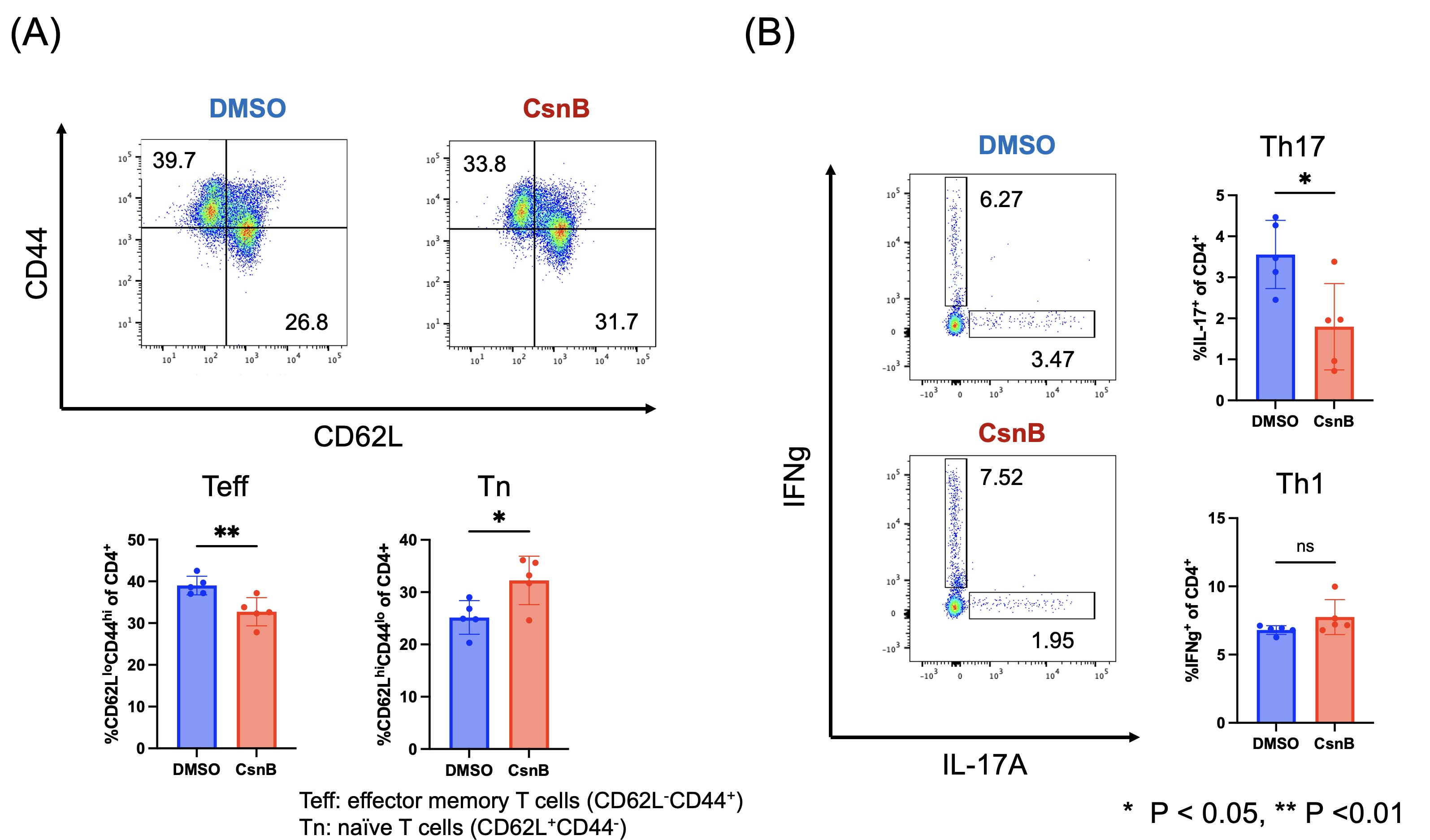
Results: Activation markers of CD4+ T cells were inhibited in flow cytometric analysis following anti-CD3/28 stimulation with CsnB. Both non-pathogenic (the condition with IL-6 and TGFbeta) and pathogenic (the condition with IL-6, IL-1beta, and IL-23) Th17 cell differentiation were significantly reduced by CsnB (Figure 1A). Phosphorylation of STAT3 was significantly inhibited by CsnB during Th17 differentiation (Figure 1B). RNA sequencing analysis revealed that CsnB decreased signaling receptor regulator activity and cytokine activity. In vivo, CsnB administration significantly attenuated arthritis development in SKG mice (Figure 2). Notably, effector memory T cells decreased, while naïve T cells increased in the treatment group (Figure 3A). Th17 cells decreased, while Th1 and Treg populations remained comparable between groups in spleen (Figure 3B). The proportion of Th17 cells was also decreased in synovium in the treatment group.
Conclusion: CsnB mitigates T cell activation and Th17 cell differentiation both in vitro and in vivo experiments. Consequently, CsnB ameliorates experimental arthritis in SKG mice. These findings underscore the potential of Nr4a1 as a promising therapeutic target of inflammatory arthritis.
To cite this abstract in AMA style:
Nakayama Y, Hiwa R, Okubo A, Shoji M, Shirakashi M, Tsuji H, Kitagori K, Nakashima R, Akizuki S, Yoshifuji H, Morinobu A. Cytosporone B, a Selective Agonist of Nr4a1, Inhibits Th17 Differentiation and Alleviates Experimental Arthritis in SKG Mice [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/cytosporone-b-a-selective-agonist-of-nr4a1-inhibits-th17-differentiation-and-alleviates-experimental-arthritis-in-skg-mice/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cytosporone-b-a-selective-agonist-of-nr4a1-inhibits-th17-differentiation-and-alleviates-experimental-arthritis-in-skg-mice/