Session Information
Date: Monday, November 13, 2023
Title: Plenary II
Session Type: Plenary Session
Session Time: 11:00AM-12:30PM
Background/Purpose: Expansion of B cell-helper T cells including T follicular helper (Tfh) and T peripheral helper (Tph) cells is a prominent feature of systemic lupus erythematosus (SLE). Tfh and Tph cells are marked by high production of the B cell chemoattractant CXCL13; however, regulation of T cell CXCL13 production and the relationship between a CXCL13+ state and other differentiated T cell states remain largely undefined.
Methods: We used mass cytometry to characterize CD4 T cell phenotypes of SLE patients (n=19) and controls (n=19). We used CRISPR screens of primary human CD4 T cells to identify transcription factors that drive core Tph phenotypes. We used transcriptomics (bulk RNA-Seq), epigenetics (ATAC-Seq and CUT&RUN), and functional studies (pharmacological modulators, lentiviral overexpression, reporter cell lines) to characterize mechanisms and pathways that influence CXCL13 production in disease relevant T cells.
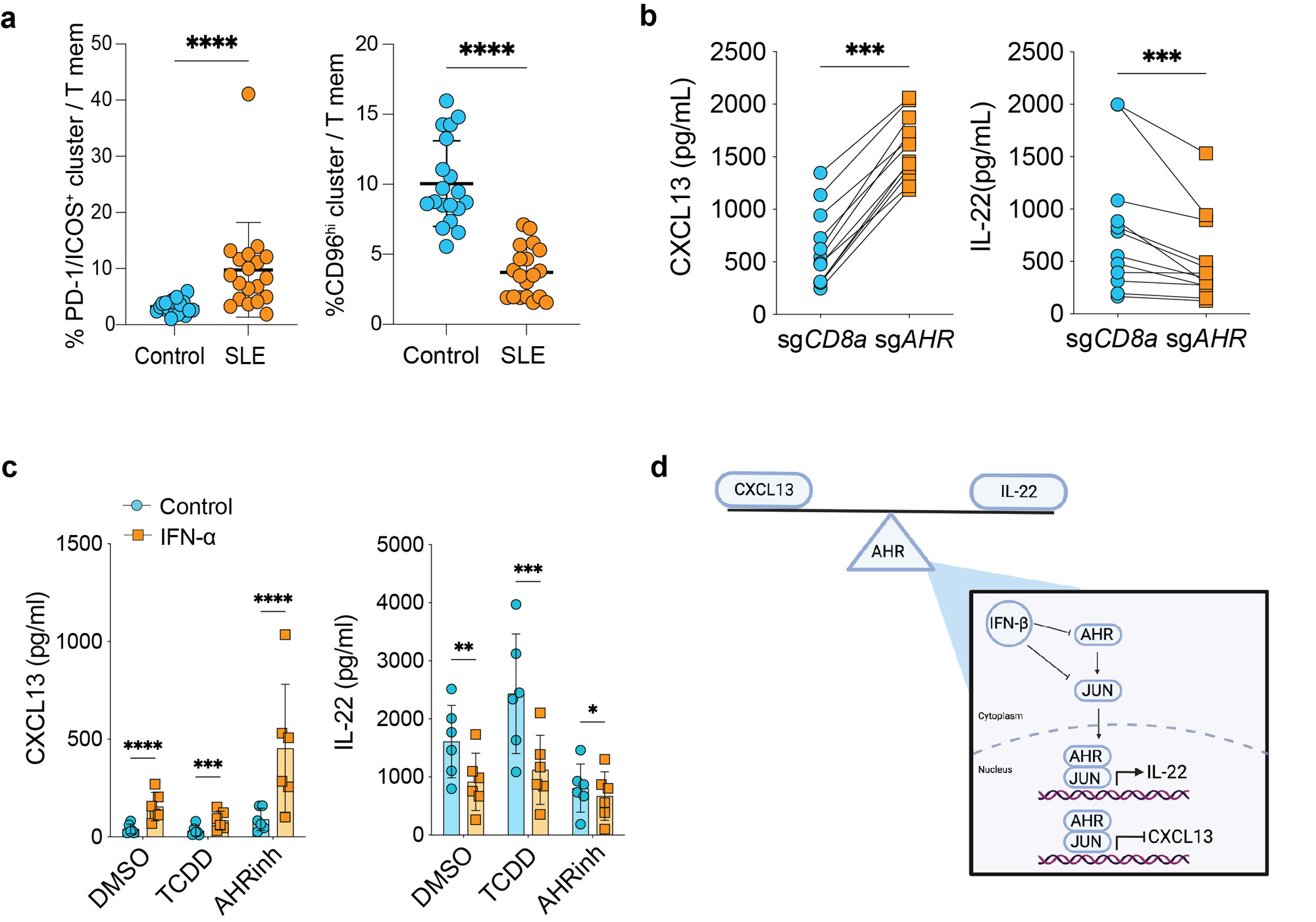
Results: Mass cytometry immunophenotyping demonstrated a dramatic imbalance in CD4 T cell phenotypes in SLE patients, with expansion of PD-1+/ICOS+ CXCL13+ T cells and reduction of CD96hi IL-22+ T cells. An arrayed CRISPR screen targeting 80 transcription factors or signaling molecules revealed that deletion of the aryl hydrocarbon receptor (AHR) significantly increased T cell CXCL13 production. Validation experiments confirmed that AHR deletion or pharmacologic inhibition strongly induced T cell CXCL13 production and T cell acquisition of a transcriptomic and epigenetic signature of ex vivo Tph cells. Conversely, AHR activation inhibited a CXCL13 production and a Tph signature while promoting an IL-22+ Th22 cell signature. Luciferase reporter assay using AHR reporter cells show decreased luciferase activity from cells cultured with SLE serum in the presence of AHR agonist. Time coursed transcriptomic analyses of AHR-activated cells combined with unbiased analysis for transcription factor binding sites in AHR CUT&RUN peaks indicated AP-1 transcription factors and AHR co-regulate gene expression. A second CRISPR screen targeting AP-1 family members identified JUN as a potent negative regulator of CXCL13 production and a Tph signature. Further CUT&RUN analyses placed both JUN and AHR at the CXCL13 locus, suggesting direct inhibition of CXCL13 expression. Finally, type I interferon (IFN), a pathogenic driver of SLE, promoted T cell CXCL13 production and a Tph-associated epigenetic signature in part by inhibiting AHR activation and reducing JUN protein expression, while overexpression of JUN inhibited the ability of IFN to induce T cell CXCL13 production.
Conclusion: Our results identify AHR and JUN as potent inhibitors of a Tph cell fate and implicate type I IFN as a key signal in SLE that inhibits AHR and JUN to induce Tph cells.
b, ELISA data for indicated cytokines in supernatants of memory CD4+ T cells nucleofected with sgAHR or sgCD8 control. All cells were cultured with TGF-beta, and each line represents a separate donor (n=12). For CXCL13 P=4.88e_4and IL_22 P=4.88e_4.
c, ELISA for CXCL13 (left) and IL_22 (right) from memory CD4+ T cells stimulated with or without IFN-alpha for 24 hours prior to addition of AHR agonist/inhibitor or DMSO control as indicated (n=6). From left to right, P-value for CXCL13: 8.5e-5, 4.05e_4, 2.9e-5, and for IL_22: 2.35e_3, 8.76e_4, 0.0338.
d, Graphical representation of proposed model in type I IFN regulation of AHR and JUN to promote Tph cells in SLE.
To cite this abstract in AMA style:
Law C, Wacleche V, Cao Y, Pillai A, Horisberger A, Bracero S, Skidanova V, Li Z, Adejoorin I, Benque I, Pena Nunez D, Simmons D, Keegan J, Chen L, Sowerby J, Medicines Partnership (AMP): RA/SLE A, Massarotti E, Costenbader K, Brenner M, Lederer J, Hultquist J, Choi J, Rao D. CXCL13+ T Cell Differentiation in Systemic Lupus Erythematosus Is Controlled by Opposing Effects of Aryl Hydrocarbon Receptor and Type I Interferon [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/cxcl13-t-cell-differentiation-in-systemic-lupus-erythematosus-is-controlled-by-opposing-effects-of-aryl-hydrocarbon-receptor-and-type-i-interferon/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cxcl13-t-cell-differentiation-in-systemic-lupus-erythematosus-is-controlled-by-opposing-effects-of-aryl-hydrocarbon-receptor-and-type-i-interferon/