Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose:
Childhood uveitis leads to sight-threatening complications in 50% of affected children and significantly impacts a child’s quality of life and function. The Multinational Interdisciplinary Working Group for Uveitis (MIWGUC) group developed outcome measures to assess pediatric uveitis, but a uveitis-specific quality patient reported outcome measure (PRO) was lacking. The Effects of Youngsters’ Eyesight on Quality of Life (EYE-Q) is the first and only validated instrument that measures vision-related quality of life and function in childhood uveitis. The EYE-Q is an easily administered, child-centered questionnaire validated in multicenter studies of >500 children in the United States. The EYE-Q was only available in the US English language. Our aim is to conduct a cross-cultural adaptation and pre-pilot testing of the EYE-Q in several languages to expand its use for global uveitis studies.
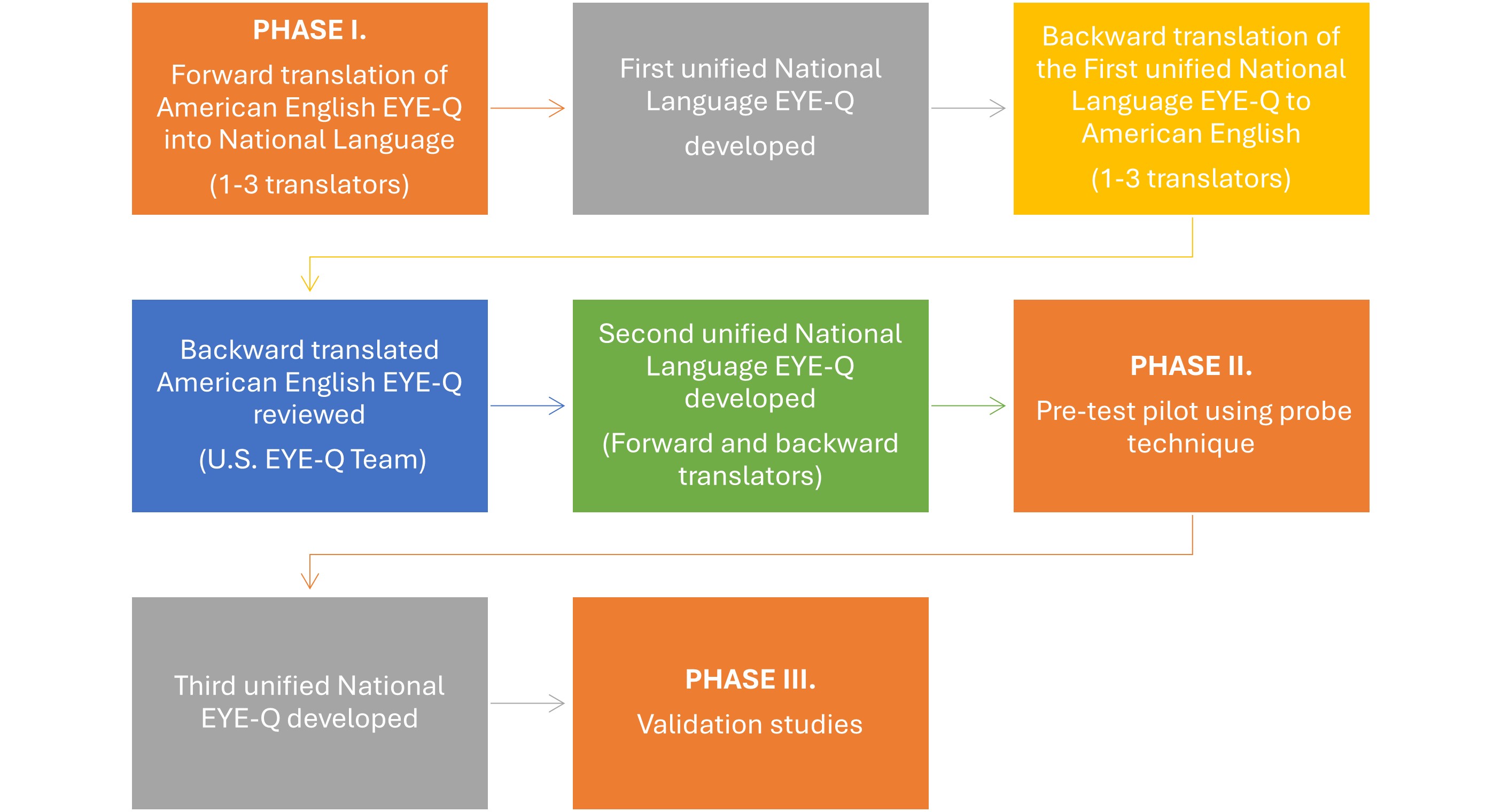
Methods: Methods were adapted from Guillemin et. al (1993, J Clin Epidemiol 46:1417–1432), similar to the CHAQ, JAMAR and CHQ – 1) Phase I: Cross-cultural adaptation and 2) Phase II: Pre-pilot testing probe (Figure 1). For Phase I, to develop the first unified version of the EYE-Q in the national language, three medical translators well-versed in American English completed a forward translation of the American English EYE-Q to the National Language. Three other medical translators then created a unified backtranslation to American English. The unified backtranslation of the EYE-Q was reviewed by the US team that developed the EYE-Q. They confirmed the relevance and comprehensibility of the items by parents and children and verified the cross-cultural equivalence versions by comparing their semantic, idiomatic, experiential and conceptual equivalencies. A second unified version of the EYE-Q was developed by the national language incorporating comments received to achieve consensus. The US team reviewed and either approved or provided additional feedback for a third review.
For Phase II, we pre-tested the second unified version of the EYE-Q in the National Language. The EYE-Q was administered to 10 children and parents to test comprehensibility and relevance of the items in each country. Each item of the translated EYE-Q had to be understood by at least 80%; items misunderstood by 20% or more of the parents or children would be reviewed and revised appropriately. If changes to the items were needed after the interviews, another round of forward and backward translations were required. This third unified version was then developed for a future Phase III Validation study.
Results: We completed forward and backward translations and developed a unified version of the EYE-Q in the following nine National languages that has undergone pre-pilot testing: Afrikaans, Croatian, Dutch, French, German, Italian, Portuguese (Portugal), Spanish (Spain) and Turkish. We are still in process of developing a unified version for Portuguese (Brazil), UK English, and Czech.
Conclusion: Cross-culturally adapted versions of the EYE-Q in several national languages have been developed. These will undergo validation in a prospective MIWGUC project to assess the correlation with other already proposed outcome measures of childhood uveitis.
To cite this abstract in AMA style:
Angeles-Han s, Anton J, Adrovic A, Costa Reis P, Boer J, Guillaume-Czitrom S, Hennard T, Jelusic M, Kasapcopur O, Maccora I, Malcova H, McDonald J, Mwase N, Simonini G, Slamang W, Toit D, Vollenhoven H, Foeldvari I. Cross-cultural Validation of the “Effects of Youngsters-Eyesight on Quality of Life (EYE-Q)”: A Measure of Vision-related Quality of Life and Function in Childhood Uveitis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/cross-cultural-validation-of-the-effects-of-youngsters-eyesight-on-quality-of-life-eye-q-a-measure-of-vision-related-quality-of-life-and-function-in-childhood-uveitis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cross-cultural-validation-of-the-effects-of-youngsters-eyesight-on-quality-of-life-eye-q-a-measure-of-vision-related-quality-of-life-and-function-in-childhood-uveitis/