Session Information
Date: Saturday, November 6, 2021
Title: Epidemiology & Public Health Poster I: COVID-19 & Vaccination (0084–0117)
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: A substantial number of patients with COVID-19 pneumonia develop a hyperinflammatory state. The anti-IL-6 receptor (IL-6R) mAb tocilizumab (TCZ) has been used in such setting. However, studies to date have had mixed results.
Our institution is unique in that nearly all services to two independent hospitals, Keck Medical Center (KMC, a private non-profit hospital owned and managed by USC) and the Los Angeles County + University of Southern California Medical Center (LAC+USC MC, a public hospital owned and managed by the County of Los Angeles), are provided by the same pool of physicians who follow similar protocols for management of COVID-19, with one crucial exception – patients admitted to LAC+USC MC do not receive TCZ, while those admitted to KMC are eligible to receive TCZ. This difference in access to TCZ results in two arms of “real-world” patients that are “quasi-randomized”, resulting in a setting that combines components of a randomized trial with real-world practice.
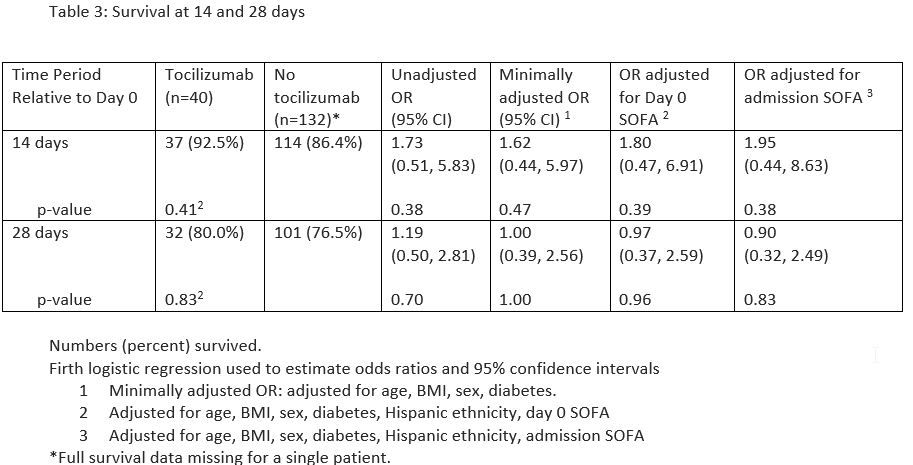
Methods: The records of patients with COVID-19 admitted to KMC or LAC+USC MC between March 1, 2020, and August 31, 2020 (COVID surge 1), and between February 20, 2021, and April 20, 2021 (COVID surge 2), were reviewed. Forty patients with severe COVID-19 pneumonia at KMC received at least a single TCZ dose (400 mg IV). These patients were matched to 133 LAC+USC MC controls on gender, age, and BMI, and, when feasible, the presence or absence of diabetes. Day 0 for KMC patients was the day of in-patient TCZ administration, and the matching day 0 for LAC+USC MC patients was the day of emergency room presentation + the number of days for which their matched KMC counterpart had been admitted prior to receiving TCZ. This numbering scheme ensured that disease timelines were similar. Outcomes were assessed at 72 hrs, day 14, and day 28. Primary outcome was ≥2-point improvement on the ordinal scale for clinical improvement (a 7-point scale based on hospitalization status, oxygen requirements, and presence of other organ support). Secondary outcome was survival at days 14 and 28.
Results: Demographics are shown in Table 1. The two populations were balanced in most baseline demographics and characteristics, although they differed in proportions of individuals of Hispanic ethnicity, presence of diabetes, and use of hydroxychloroquine for treatment of COVID-19. Our findings failed to demonstrate improved clinical outcomes among patients receiving vs not receiving TCZ at any of the time points (Table 2). Moreover, there was also no difference between these cohorts in survival at days 14 and 28 (Table 3).
Conclusion: While our two hospitals are separate, the same physicians and similar treatment protocols (other than TCZ use) resulted in a natural experimental setting. In our study, use of TCZ for COVID-19 pneumonia did not lead to a significant benefit in this quasi-randomized, real-world environment. Moreover, clinical outcomes of patients in our public hospital were no worse than those in our private hospital. The hyperinflammatory state of severe COVID-19 is unlikely to be driven by a single inflammatory cytokine, and the risks of using any and all types of immunosuppression must be carefully weighed against the perceived benefits.
To cite this abstract in AMA style:
Wise L, Mathias L, Mack W, Kafi A, Kothari Y, Rao O, Stohl W. COVID-19 Pneumonia in Two Hospitals: Similar Outcomes Despite Differential Use of Tocilizumab [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/covid-19-pneumonia-in-two-hospitals-similar-outcomes-despite-differential-use-of-tocilizumab/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/covid-19-pneumonia-in-two-hospitals-similar-outcomes-despite-differential-use-of-tocilizumab/