Session Information
Date: Saturday, November 16, 2024
Title: RA – Treatment Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Existing American College of Rheumatology (ACR) and European Alliance of Associations for Rheumatology (EULAR) guidelines regarding medication tapering in patients with rheumatoid arthritis (RA) in low disease activity or remission are based on low-level evidence. In our previous study within the Rheumatoid Arthritis Medication Tapering (RheuMTAP) cohort1 of patients with well-controlled RA on a biologic disease-modifying antirheumatic drug or targeted synthetic disease-modifying antirheumatic drug (bDMARD/tsDMARD), we found that tapering or stopping the bDMARDs/tsDMARDs or both conventional disease-modifying antirheumatic drug (csDMARD) and bDMARDs/tsDMARDs increased risk of flare several fold compared to continuing medication, and that tapering of csDMARDs alone had a much lower risk of flare compared with tapering bDMARDs/tsDMARDs. In this study, we analyzed cost savings of medication tapering in Highmark insured patients within the RheumMTAP cohort.
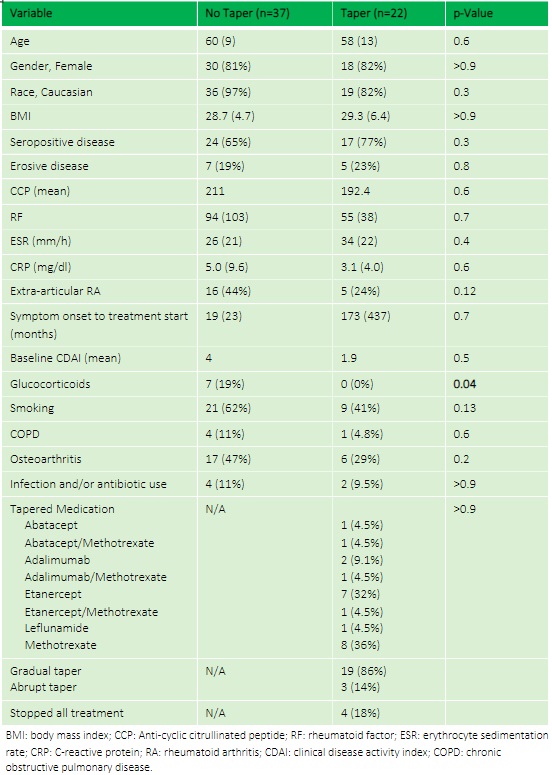
Methods: A prospective cohort of patients with RA in sustained remission or low disease activity while on stable biologics/DMARDs for at least 6 months underwent medication tapering and were longitudinally followed for 2 years (RheuMTAP cohort, 11/2018-11/2020). Eligibility criteria for each patient were validated via a manual electronic health record (EHR) review. In this study, the subset of patients with Highmark insurance were included to compare healthcare per member per month (PMPM) costs and flare rates between patients whose medications were tapered versus not tapered. Flare was defined as provider-diagnosed worsening of CDAI >10 and/or any worsening of disease activity leading to initiation/change/increase of therapy i.e. rescue therapy.
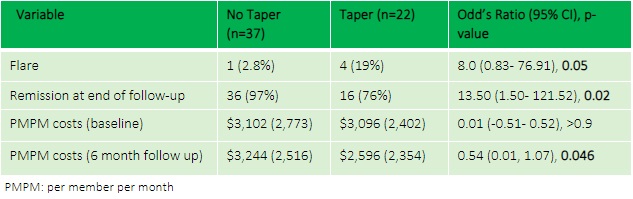
Results: The RheuMTAP cohort included a total of 131 patients with stable RA on a DMARD or biologic medication. Of these, 59 patients met eligibility criteria with Highmark insurance of which 22 patients underwent medication tapering. The most frequently tapered biologics were Etanercept (32%) and Adalimumab (9.1%), and the most frequently tapered csDMARD was methotrexate (36%). Mean PMPM costs reduced from $3,096 at baseline to $2,596 at 6 months after tapering in the taper group compared to an increase from $3,102 at baseline to $3,244 at 6 months in the no-taper group (between group 6-month follow-up OR 0.54, 95% CI 0.01- 1.07, p=0.046) with a difference-in-differences calculation showing savings of $735 (p=0.19). Flare rate was higher in the taper group compared to no-taper (19% vs 2.8%, OR 8, 95% CI 0.83- 76.91, p=0.05).
Conclusion: In our study analyzing costs and clinical outcomes of tapering medications in stable RA, there was a statistically significant reduction in PMPM costs for patients who tapered or stopped biologics/csDMARDs, as well as lower costs compared to baseline prior to medication tapering. However, the cost reduction came at the expense of a higher flare rate in the taper group especially in the subset of patients tapering their biologic therapy. We plan to expand our cohort to a multicenter study to analyze clinical outcomes and costs of methotrexate tapering strategies, and to identify biomarkers predictive of successful tapering.
1 Tageldin et al. Rheumatology. Oct 2023
To cite this abstract in AMA style:
Hajizadeh S, Barrett T, Yin Y, Sharma D. Costs and Clinical Outcomes of the Rheumatoid Arthritis Medication Tapering Cohort [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/costs-and-clinical-outcomes-of-the-rheumatoid-arthritis-medication-tapering-cohort/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/costs-and-clinical-outcomes-of-the-rheumatoid-arthritis-medication-tapering-cohort/