Session Information
Date: Tuesday, November 9, 2021
Title: T Cell Biology & Targets in Autoimmune & Inflammatory Disease Poster (1507–1515)
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: Current therapies for autoimmune disease often lead to treatment-limiting immunosuppression. Selective manipulation of antigen (Ag)-specific immune responses could enhance our therapeutic approach. Targeting members of the NR4A family of nuclear receptors could achieve this as they mediate immune tolerance in Ag-activated lymphocytes. Thymic deletion of multiple – but not individual – NR4A genes (Nr4a1 and Nr4a3 > > Nr4a2, which is minimally expressed) results in deficiency of regulatory T cells (Treg) and a severe inflammatory disease (Fig. 1A-B). Thus, it has been challenging to unmask additional redundant functions of these druggable transcription factors (TFs) in conventional T cells (Tconv). We devised innovative conditional genetic and bone marrow chimera strategies to preserve Treg homeostasis and overcome this obstacle.
Methods: We created mixed radiation bone marrow (BM) chimeras reconstituted with congenically marked CD45.2 Nr4a1-/-Nr4a3-/- (germline double knock-out or gDKO) and CD45.1 wild-type (WT) bone marrow. Control chimeras were reconstituted with CD45.2 WT and CD45.1 WT BM. We evaluated Tconv and Treg homeostasis and thymic development with conventional methods.
Results: In DKO:WT chimeras, the Treg compartment was reconstituted from largely WT donor cells (Fig. 1D). Despite this, chimeras rapidly developed anti-nuclear autoantibodies (ANA) and evidence of cell-extrinsic polyclonal B cell activation (Fig. 1E-G). CD4 and CD8 single positive (SP) thymocytes accumulate in DKO but not control chimeras, suggesting a profound cell-intrinsic impairment of negative selection of DKO thymocytes (Fig 2A-D). Supporting this, activated caspase-3 expression was reduced in DKO double-positive (DP) thymocytes (Fg 2C-D). In addition, peripheral DKO CD8 T cells with a memory phenotype (CD44hi) accumulate in DKO:WT chimeras (Fig 2E-G), but not in CD8-cre Nr4a1fl/fl Nr4a3-/- mice which delete NR4A genes only after thymic selection in the CD8 SP stage (Fig 2H-J). DKO CD4 Tconv cells expressing phenotypic markers of anergy (FR4hiCD73hi) accumulate in these DKO:WT chimeras (Fig. 3A-B), suggesting escape of self-reactive T cells into the periphery. Indeed, these cells indeed exhibit a dampened proximal signaling downstream of the T cell receptor (TCR) (Fig 3C-E). However, these self-reactive DKO CD4 Tconv also exhibit exaggerated IL-2 production (Fig 3F-G), suggesting that functional anergy is defective.
Conclusion: Together, these data support the unifying hypothesis that NR4A family members play cell-intrinsic, but redundant, roles in both central and peripheral CD4 T cell tolerance. These studies reveal roles for the NR4A family in multiple layered T cell tolerance mechanisms and demonstrate that each is essential to preserve immune homeostasis.
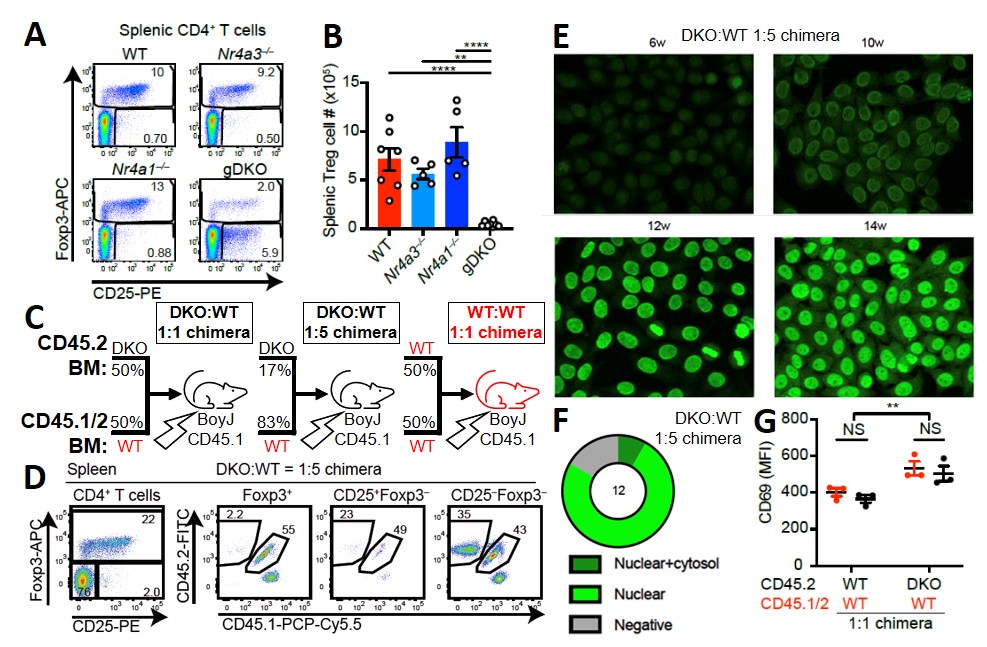 Figure 1. DKO:WT chimeras develop ANA and systemic autoimmunity despite restored Treg homeostasis. A) Representative plots showing loss of Foxp3+ Treg in germline DKO (gDKO) mice. B) Quantification of data from A. C) Schematic of mixed bone marrow chimera design. D) Representative plots from 1:5 DKO:WT chimeras showing reconstitution of Foxp3+ Treg compartment by WT donor cells. E) Representative immunofluorescence images on serum of 1:5 DKO:WT chimeras showing development of ANA in sera between 6_14 weeks post-reconstitution. F) Penetrance and staining pattern of ANA from 1:5 DKO:WT chimeras. G) Both DKO and WT B cells from DKO:WT but not WT:WT chimeras show increased CD69 expression.
Figure 1. DKO:WT chimeras develop ANA and systemic autoimmunity despite restored Treg homeostasis. A) Representative plots showing loss of Foxp3+ Treg in germline DKO (gDKO) mice. B) Quantification of data from A. C) Schematic of mixed bone marrow chimera design. D) Representative plots from 1:5 DKO:WT chimeras showing reconstitution of Foxp3+ Treg compartment by WT donor cells. E) Representative immunofluorescence images on serum of 1:5 DKO:WT chimeras showing development of ANA in sera between 6_14 weeks post-reconstitution. F) Penetrance and staining pattern of ANA from 1:5 DKO:WT chimeras. G) Both DKO and WT B cells from DKO:WT but not WT:WT chimeras show increased CD69 expression.
 Figure 2. DKO thymocytes have a cell-intrinsic defect in negative selection. A) Key for graphs in B, F, and G. B) Ratio of CD45.2:CD45.1/2 cells among double positive (DP) and CD4 and CD8 single positive (SP) thymocytes normalized to DP. C) Representative plots showing reduced activated caspase_3 expression in DKO DP thymocytes from 1:1 DKO:WT chimeras upon ex vivo stimulation with anti-CD3. D) Quantification of data from C. E) Representative plots showing accumulation of CD44hi CD8 T cells from DKO chimeras. F) Quantification of data from E at 6 or 10_12 weeks post-reconstitution. G) Ratio of CD45.2:CD45.1/2 CD8+CD44hi cells. H) Representative plots showing Nur77 expression in thymocytes from CD8-cre and CD8-cre Nr4a1fl/fl Nr4a3-/- (CD8-cre cDKO) mice. I) Quantification of Nur77 expression in thymocytes (as in H) and splenocytes (key as in H). J) CD8+ CD44hi cells from CD8-cre and CD8-cre cDKO mice.
Figure 2. DKO thymocytes have a cell-intrinsic defect in negative selection. A) Key for graphs in B, F, and G. B) Ratio of CD45.2:CD45.1/2 cells among double positive (DP) and CD4 and CD8 single positive (SP) thymocytes normalized to DP. C) Representative plots showing reduced activated caspase_3 expression in DKO DP thymocytes from 1:1 DKO:WT chimeras upon ex vivo stimulation with anti-CD3. D) Quantification of data from C. E) Representative plots showing accumulation of CD44hi CD8 T cells from DKO chimeras. F) Quantification of data from E at 6 or 10_12 weeks post-reconstitution. G) Ratio of CD45.2:CD45.1/2 CD8+CD44hi cells. H) Representative plots showing Nur77 expression in thymocytes from CD8-cre and CD8-cre Nr4a1fl/fl Nr4a3-/- (CD8-cre cDKO) mice. I) Quantification of Nur77 expression in thymocytes (as in H) and splenocytes (key as in H). J) CD8+ CD44hi cells from CD8-cre and CD8-cre cDKO mice.
 Figure 3. Conventional DKO CD4+ T cells have impaired peripheral tolerance. A) Representative plots from 1:5 DKO:WT chimeras showing gating strategy to identify FR4hiCD73hi anergic phenotype CD4 T cells among naïve and memory compartments. B) Ratio of CD45.2:CD45.1/2 FR4hiCD73hi cells from 1:5 DKO:WT chimeras in naïve (left) and memory (right) compartments 6_12 weeks post-reconstitution. C) Representative histograms showing intracellular phospho-Erk staining after ex vivo stimulation of non-anergic, intermediate anergic, and anergic phenotype cells from 1:5 DKO:WT chimeras in both naïve and memory compartments, gated as depicted in A. D-E) Quantification of data shown in C. F) Intracellular staining for IL_2 after ex vivo stimulation of splenocytes from 1:1 DKO:WT chimeras with anti-CD3 followed by PMA and ionomycin. G) Quantification of data shown in F.
Figure 3. Conventional DKO CD4+ T cells have impaired peripheral tolerance. A) Representative plots from 1:5 DKO:WT chimeras showing gating strategy to identify FR4hiCD73hi anergic phenotype CD4 T cells among naïve and memory compartments. B) Ratio of CD45.2:CD45.1/2 FR4hiCD73hi cells from 1:5 DKO:WT chimeras in naïve (left) and memory (right) compartments 6_12 weeks post-reconstitution. C) Representative histograms showing intracellular phospho-Erk staining after ex vivo stimulation of non-anergic, intermediate anergic, and anergic phenotype cells from 1:5 DKO:WT chimeras in both naïve and memory compartments, gated as depicted in A. D-E) Quantification of data shown in C. F) Intracellular staining for IL_2 after ex vivo stimulation of splenocytes from 1:1 DKO:WT chimeras with anti-CD3 followed by PMA and ionomycin. G) Quantification of data shown in F.
To cite this abstract in AMA style:
Hiwa R, Nielsen H, Mueller J, Zikherman J. Control of T Cell Tolerance by the NR4A Family of Nuclear Receptors [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/control-of-t-cell-tolerance-by-the-nr4a-family-of-nuclear-receptors/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/control-of-t-cell-tolerance-by-the-nr4a-family-of-nuclear-receptors/
