Session Information
Date: Sunday, November 13, 2022
Title: Systemic Sclerosis and Related Disorders – Clinical Poster II
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
Background/Purpose: In the randomized placebo-controlled SENSCIS trial in patients with SSc-ILD, nintedanib reduced the rate of decline in forced vital capacity (FVC) (mL/year) over 52 weeks by 44%, with adverse events that were manageable for most patients. SENSCIS-ON is an open-label extension study investigating adverse events and decline in FVC in patients with SSc-ILD treated with nintedanib over the longer term.
Methods: In the SENSCIS trial, patients received nintedanib or placebo until the last patient reached week 52 but for ≤100 weeks. Patients with SSc-ILD who completed the SENSCIS trial or a drug–drug interaction (DDI) study of nintedanib and oral contraceptive in which female patients with SSc-ILD received nintedanib for ≥14 days to approximately 28 days were eligible to enter SENSCIS-ON. We analyzed adverse events and changes from baseline in FVC (mL) over 148 weeks in patients who received nintedanib in SENSCIS and continued nintedanib in SENSCIS-ON (“continued nintedanib” group) and in patients who received placebo in SENSCIS or who received nintedanib for ≤28 days in the DDI study (“initiated nintedanib” group). Analyses were descriptive. Analyses of FVC were based on observed data available at the respective time-point.
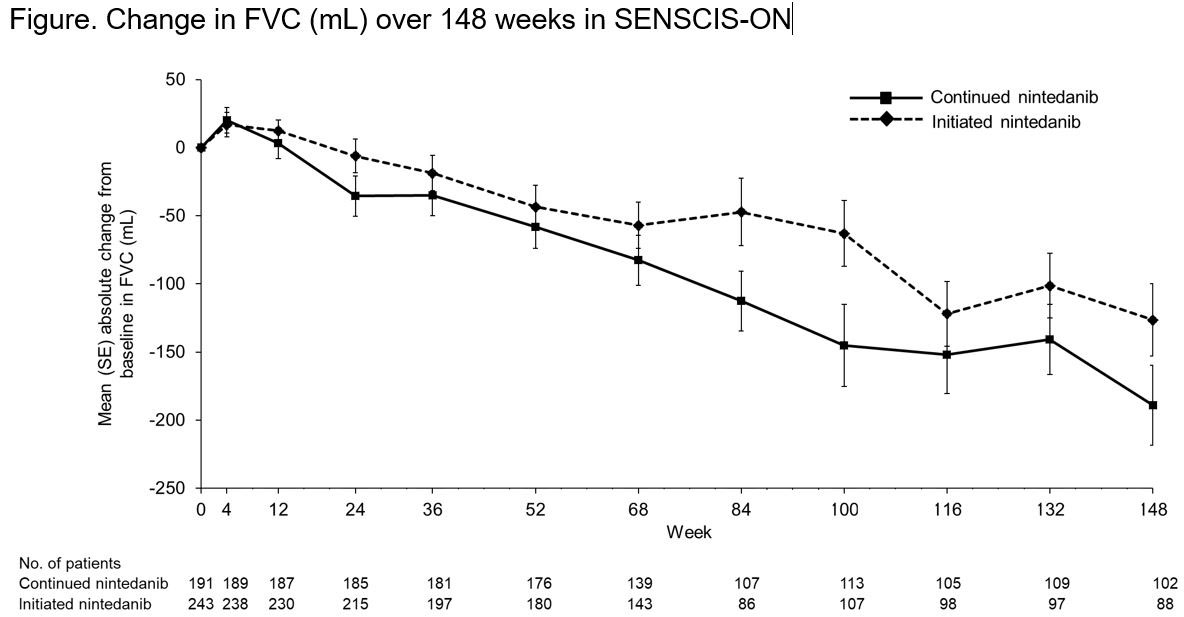
Results: The continued nintedanib group comprised 197 patients and the initiated nintedanib group comprised 247 patients (231 from SENSCIS, 16 from the DDI study). In these groups, respectively, mean (SD) FVC at the start of SENSCIS-ON was 2379 (754) mL and 70.4 (18.1) % predicted and 2443 (814) mL and 70.8 (17.9) % predicted. In total, 126 (64.0%) and 125 (50.6%) patients in the continued nintedanib and initiated nintedanib groups, respectively, were still receiving nintedanib at week 148 of SENSCIS-ON. Diarrhea was the most frequent adverse event (Table). Serious adverse events were reported in 76 (38.6%) patients in the continued nintedanib group and 95 (38.5%) patients in the initiated nintedanib group. Among patients who continued nintedanib and initiated nintedanib, respectively, 53 (26.9%) and 148 (59.9%) had ≥1 dose reduction and 72 (36.5%) and 131 (53.0%) had ≥1 treatment interruption. Adverse events led to discontinuation of nintedanib in 29 (14.7%) patients in the continued nintedanib group and 72 (29.1%) patients in the initiated nintedanib group. Mean (SE) changes in FVC from baseline to week 148 of SENSCIS-ON were −189.1 (29.5) mL in the continued nintedanib group and −126.4 (26.4) mL in the initiated nintedanib group (Figure).
Conclusion: The safety profile of nintedanib over 148 weeks of SENSCIS-ON was consistent with that reported in the SENSCIS trial, characterized mainly by gastrointestinal events that were manageable for most patients. These results support the potential use of nintedanib in the long-term treatment of patients with SSc-ILD.
To cite this abstract in AMA style:
Allanore Y, Vonk M, Distler O, Azuma A, Mayes M, James A, Kohlbrenner V, Alves M, Khanna D, Highland K. Continued Treatment with Nintedanib in Patients with Systemic Sclerosis-Associated Interstitial Lung Disease (SSc-ILD): Three-Year Data from SENSCIS-ON [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/continued-treatment-with-nintedanib-in-patients-with-systemic-sclerosis-associated-interstitial-lung-disease-ssc-ild-three-year-data-from-senscis-on/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/continued-treatment-with-nintedanib-in-patients-with-systemic-sclerosis-associated-interstitial-lung-disease-ssc-ild-three-year-data-from-senscis-on/