Session Information
Date: Sunday, October 26, 2025
Title: (0430–0469) Rheumatoid Arthritis – Diagnosis, Manifestations, and Outcomes Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Low disease activity has been identified as a treatment goal for the treatment of RA. A singlecenter RA registry (BRASS) reported a difference in outcome in RA pts who achieved remissionversus low disease activity 1. When LDA was subdivided between very low disease activity(VLDA >2.8< 6) and LDA scores from >6< 10 and remission CDAI < 2.8 there were alsodifferences in outcome between those pts in VLDA versus LDA suggesting that the threshold for“true” LDA should be lowered. This study further examined this issue in a large US RA registry
Methods: Population: RA patients from the US-based CorEvitas RA registry (122 private sites, 21academic sites).Inclusion: Patients (pts) on a biologic (baseline) and a 1-year (9-15 mos) and 2-year (21-27mos). Target defined as LDA (CDAI ≤10). Pts at one year divided into 1) Consistently on target2) Lose target; 4) Gain Target 5) Consistently not at target. The sub-population consistently ontarget were divided into remission (≤2.8), very low disease activity (VLDA: >2.8 and ≤6) or highLDA (HLDA >6 and ≤10) at one year. Baseline characteristics, HAQdi, pain and fatigue at 1and 2 years were compared across groups. Linear regression analysis examined these outcomesat 1 year adjusting for age, gender, RA duration, Charlson Comorbidity Index(CCI) and CDAI(all at baseline).
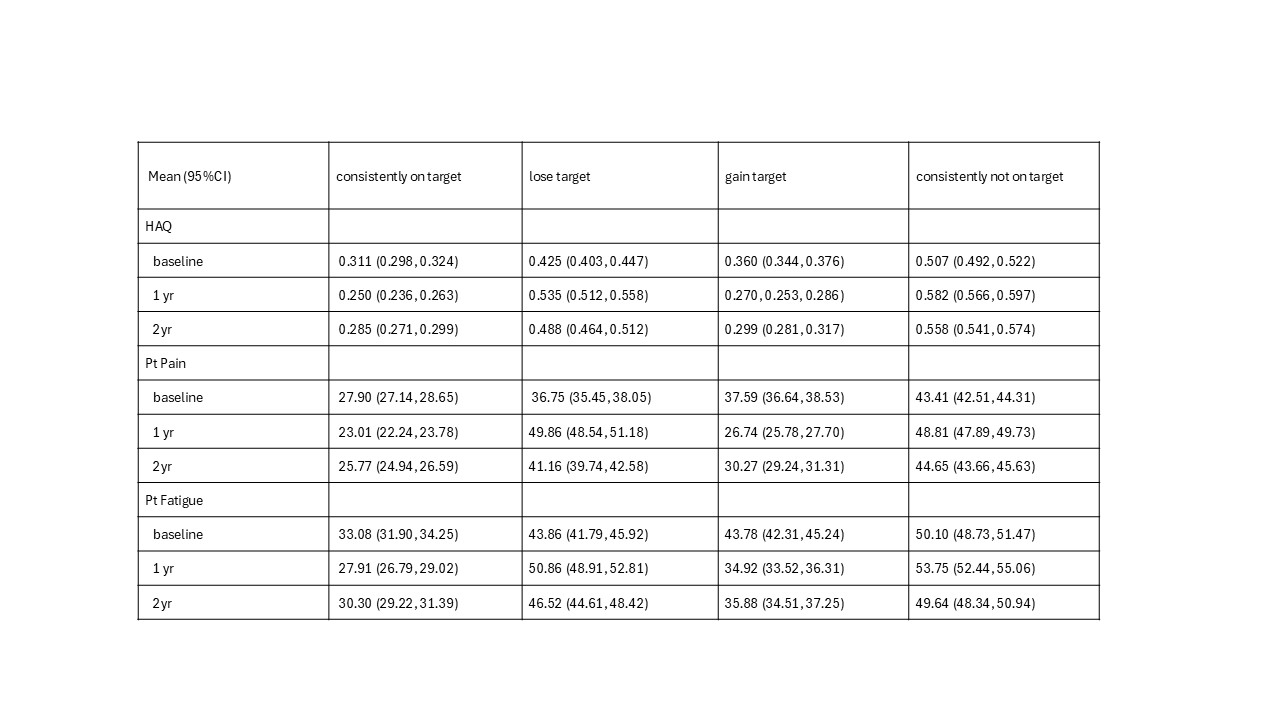
Results: 13,513 pts with baseline visits between 2001-2023 were included in the analysis. 5,570 (41.4%)“consistently at target”,1,330 (9.8%) “Flare/Lose Target”, 2,542 (18.8%) “Gain Target” , and4,071 (30.13%) “Consistently not at Target” .“Consistently at Target”, 2,690 (48.3%) pts were classified at one year as “Remission” , 1,694(30.4%) VLDA , and 1,186 (21.3% ) HLDA. Baseline characteristics are seen in Table 1.Groups with CDAI > 10 at 1-year had worse functional outcome in HAQdi, pt reported painand fatigue at both year 1 and 2 than the two groups who had CDAI < 10 at 1-year in bothunadjusted and adjusted models (p< 0.01).Within the consistently at target group, HLDA (CDAI >6 and < 10) pts had significantly worsefunctional outcome on HAQdi, pt reported pain, and fatigue than the VLDA and remissiongroups at year 1 and 2 years (p< 0.01). The VLDA patients had worse functional outcome thanthe remission group (p< 0.01). Figure 1.
Conclusion: This very large observational study demonstrates that there are meaningful patient-reporteddifferences in the consistency of the categories of “LDA” response including HAQdi, pt pain andfatigue, that are significantly associated with LDA subcategory status (remission, VLDA andHLDA). These significant and meaningful differences in “LDA status” with treatment are lostwhen considering only a broad definition of LDA. Our results show that the LDA cutpointshould be lowered from CDAI ≤10 to CDAI ≤6 for a more realistic evaluation of patientfunctional outcomes when judging what is now called LDA.
To cite this abstract in AMA style:
Kremer J, Kane K, Reed G, Weinblatt M. Consistency of CDAI Low-Disease Activity (LDA) Outcomes with Time in a Large US Registry: Association With Patient-Reported Clinical Outcomes and Measures of Quality of Life [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/consistency-of-cdai-low-disease-activity-lda-outcomes-with-time-in-a-large-us-registry-association-with-patient-reported-clinical-outcomes-and-measures-of-quality-of-life/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/consistency-of-cdai-low-disease-activity-lda-outcomes-with-time-in-a-large-us-registry-association-with-patient-reported-clinical-outcomes-and-measures-of-quality-of-life/

.jpg)
.jpg)