Session Information
Date: Friday, November 6, 2020
Title: SLE – Diagnosis, Manifestations, & Outcomes Poster I: Clinical Manifestations
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Patient Reported Outcomes (PROs) can provide critical data in measuring the impact of a disease on an individual as well as the quality of response to treatment. However, PROs are oftentimes recorded intermittently and require patients to recall a period of several weeks or months. As a result, important PRO information may not always be representative of the complete recall period. The development of mobile technology to collect PRO data in an electronic format (ePRO) allows for more frequent assessment in real time and in the person’s regular environment. The goal of this study was to assess the compliance and equivalence of a smart phone application (app) in collecting PRO data in individuals SLE.
Methods: A smart phone app was developed that collects ePRO data from various instruments, including the Fatigue Severity Scale (FSS), Functional Assessment of Chronic Illness Therapy-Fatigue Scale (FACIT-F), Short Form Health Survey (SF-36), Patient Reported Outcome Measurement Information System (PROMIS-29), Systemic Lupus Activity Questionnaire (SLAQ), and LupusPRO. Additionally, the duration of morning stiffness as well as patient global assessment (PtGA), pain and fatigue using a 10 cm visual analog scale were collected. Information was collected as part of a multicenter randomized interventional clinical trial of patients who met ACR criteria for the classification of SLE (NCT03098823). ePRO information was collected daily (PtGA, fatigue, pain, morning stiffness), 5-days per week (FSS, FACIT-F), or weekly (SF-36, PROMIS-29, SLAQ, LupusPRO), and biometric data (steps, sleep and location) was recorded daily with the app or a linked smart watch. Additionally, all PRO instruments were completed on seven occasions at the clinical sites utilizing both the app and standard paper forms separated by a distraction. To determine agreement between information collected with the app and the paper-administered PROs, intra-class correlation coefficients (ICCs), paired student’s t-tests, and Bland-Altman plots for data collected at each of the seven site visits were evaluated. Compliance, demographic information and Cronbach’s alpha as a measure of survey reliability were also assessed.
Results: For the 62 subjects from diverse ancestral backgrounds, compliance with ePRO completion was high for all PRO surveys ( >75%; Figure 1). Cronbach’s alpha values for each PRO ranged from 0.74 to 0.96 for the ePROs, and 0.70 to 0.96 for the paper versions, suggesting moderate to high inter-survey reliability in measuring the targeted concepts. Of the 168 ICC values computed from each site visit for each survey, 41 were indicative of moderate (0.5 to 0.75), 69 good (0.75 to 0.9) and 55 excellent ( >0.90) reliability between measurement methods (Table 1). Bland-Altman plots verified method agreement with 151 of the pairwise comparisons yielding an insignificant difference.
Conclusion: The high rate of compliance in collecting ePROs and the high level of consistency between data collected by paper and electronic methods of administration indicate that the app provides highly reliable information capable of cataloging real-time changes in PROs in SLE patients.
 Compliance with PRO instruments collected with a mobile app.
Compliance with PRO instruments collected with a mobile app.
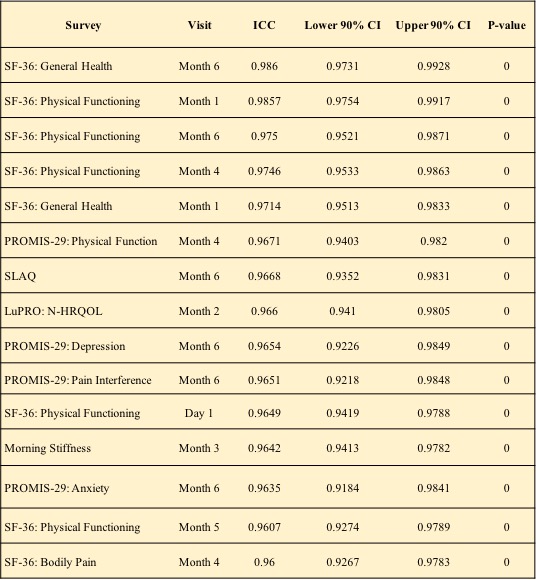 Top 15 intraclass correlation coefficients (ICCs) and respective 90% confidence intervals.
Top 15 intraclass correlation coefficients (ICCs) and respective 90% confidence intervals.
To cite this abstract in AMA style:
Bell K, Dykas C, Rainey H, Comberg M, Mora M, Lipsky P. Compliance and Validation of Patient Reported Outcome Information Collected from Lupus Patients Using a Mobile Application [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/compliance-and-validation-of-patient-reported-outcome-information-collected-from-lupus-patients-using-a-mobile-application/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/compliance-and-validation-of-patient-reported-outcome-information-collected-from-lupus-patients-using-a-mobile-application/
