Session Information
Date: Monday, November 9, 2020
Title: T Cell Biology & Targets in Autoimmune & Inflammatory Disease (2043–2047)
Session Type: Abstract Session
Session Time: 10:00AM-10:50AM
Background/Purpose: CD4+ T cells are implicated in the pathogenesis of rheumatoid arthritis (RA) due to strong genetic association with HLA class II alleles, the presence of CD4+ T cells in the rheumatoid joint and the efficacy of T cell directed therapies. In addition, CD4+ T cells specific to citrullinated epitopes are increased in RA and their frequency is influenced by disease duration and by biologic therapy. However, technical challenges due to the rarity of citrulline (cit)-specific T cells have limited our understanding of how T cell phenotype and specificity can vary within and between individuals. To address this gap in knowledge, we characterized the frequency and phenotype of cit-specific CD4+ T cells in a cross-sectional cohort of RA subjects using multiplex HLA class II tetramer staining combined with our new computational approach for phenotyping rare cell populations.
Methods: The cohort consisted of 77 seropositive RA subjects and 30 healthy control subjects matched for age, sex and race. All RA subjects met the 2010 American College of Rheumatology criteria, and had at least one HLA-DRB1*04:01 allele, and were ACPA positive. The multiplex HLA class II tetramer assay stained six distinct groups of epitope specificities: alpha-enolase, aggrecan, cartilage intermediate layer protein, a combined vimentin and fibrinogen peptide pool with influenza included as a reference for comparison to a known vaccine response. To facilitate unbiased assessment of the phenotypic distribution of rare, autoreactive T cells within and across subjects, we first clustered total CD4+ T cells from each individual using Phenograph, and then performed hierarchical metaclustering to align clusters between individuals. The relationship between clinical variables and cell frequencies was modeled using quasibinomial generalized linear models with age and sex as covariates.
Results: Consistent with our previous study analyzing single antigen specificities, the frequency of cit-specific CD4+ T cells was significantly increased in RA subjects. Notably, individual RA subjects had CD4+ T cells specific for multiple cit-specific antigens, yet the predominant specificities varied between individuals. Furthermore, the predominant immunophenotype of cit-specific CD4+ T cells varied based on antigen specificity. Importantly, we also observed significant associations between immunophenotype and disease duration and disease activity for individual antigen specificities.
Conclusion: Here, we show that there is not a single dominant specificity or cellular phenotype present in individuals with RA. Instead, the immune response to cit-antigens targets multiple antigens with a broad range of phenotypes and these features are dynamic, which parallels reported characteristics of ACPA responses during development of RA. Serologic studies have demonstrated an expansion of frequency and targets of ACPA prior to RA development. Our findings suggest that a similar process may occur for cit-specific CD4+ T cells, perhaps initiated by failed tolerance to a single initiating cit-antigen years prior to disease onset, followed by an expansion of the T cell response to multiple cit-antigens.
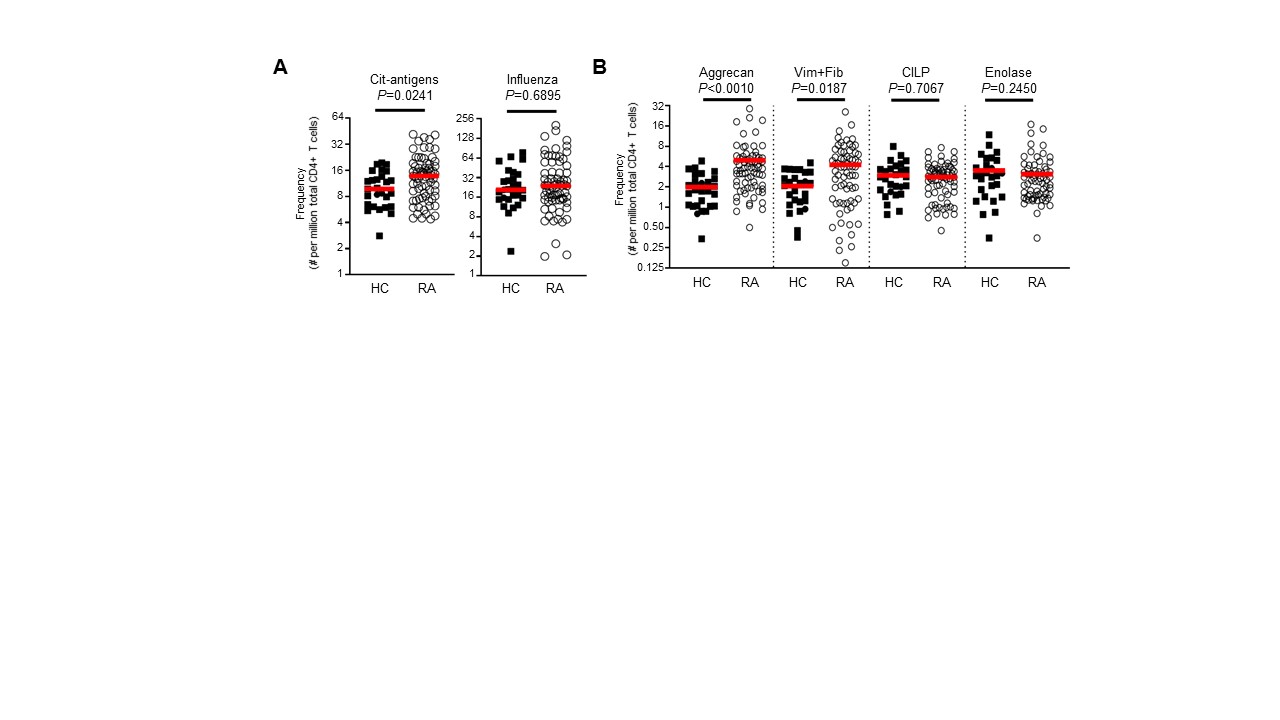 Figure 1. Increased frequency of citrulline specific CD4 T cells in individuals with rheumatoid arthritis (RA) compared to healthy control (HC) subjects. (A) Ex vivo frequencies of T cells for all cit-antigens combined, and for influenza antigen. (B) Ex vivo frequencies of T cells specific for each cit-antigen specificity individually. For A and B, symbols represent individual subjects (n=30 for HC; n=77 for RA), and horizontal bars show the median. P-values were calculated with an unpaired non-parametric Mann-Whitney test.
Figure 1. Increased frequency of citrulline specific CD4 T cells in individuals with rheumatoid arthritis (RA) compared to healthy control (HC) subjects. (A) Ex vivo frequencies of T cells for all cit-antigens combined, and for influenza antigen. (B) Ex vivo frequencies of T cells specific for each cit-antigen specificity individually. For A and B, symbols represent individual subjects (n=30 for HC; n=77 for RA), and horizontal bars show the median. P-values were calculated with an unpaired non-parametric Mann-Whitney test.
To cite this abstract in AMA style:
James E, Muir V, Rims C, Uchtenhagen H, Hocking A, Posso S, Bukiri H, Carlin J, Ng B, Linsley P, Buckner J. Complex, Dynamic Attributes of Antigen-specific T Cells in Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/complex-dynamic-attributes-of-antigen-specific-t-cells-in-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/complex-dynamic-attributes-of-antigen-specific-t-cells-in-rheumatoid-arthritis/


