Session Information
Date: Saturday, November 7, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster II
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: There is no single accepted measure of low/minimal disease activity (LDA/MDA) for patients with PsA; thus, describing the characteristics of real-world patients meeting different criteria will provide insight as to the most appropriate criteria for assessing disease control. The aim of this study was to identify and characterize patients with PsA who met LDA/MDA and remission/very low disease activity (VLDA) criteria by multiple measures of PsA disease severity.
Methods: This was a cohort study of patients (≥18 years) who enrolled in the Corrona PsA/SpA Registry between March 2013 and March 2020. Patients who received a biologic DMARD or Janus kinase inhibitor at enrollment, had a 6-month follow-up visit, and were not in a state of LDA/MDA at baseline were included. Percentages of patients who achieved LDA/MDA, remission/VLDA, and Clinical Disease Activity Index for Psoriatic Arthritis (cDAPSA) thresholds at 6-month follow-up were identified. We also examined the patient acceptable symptom state (PASS) for Patient Global Assessment (PtGA) and PtGA of Psoriasis and Arthritis (PtGA PA) using published cutoffs and examined how these exploratory thresholds compared with MDA, VLDA, and cDAPSA thresholds at 6 months. Clinical characteristics were assessed in patients who met disease thresholds at 6 months. Continuous measures were reported as means and standard deviations and categorical measures as frequencies and percentages.
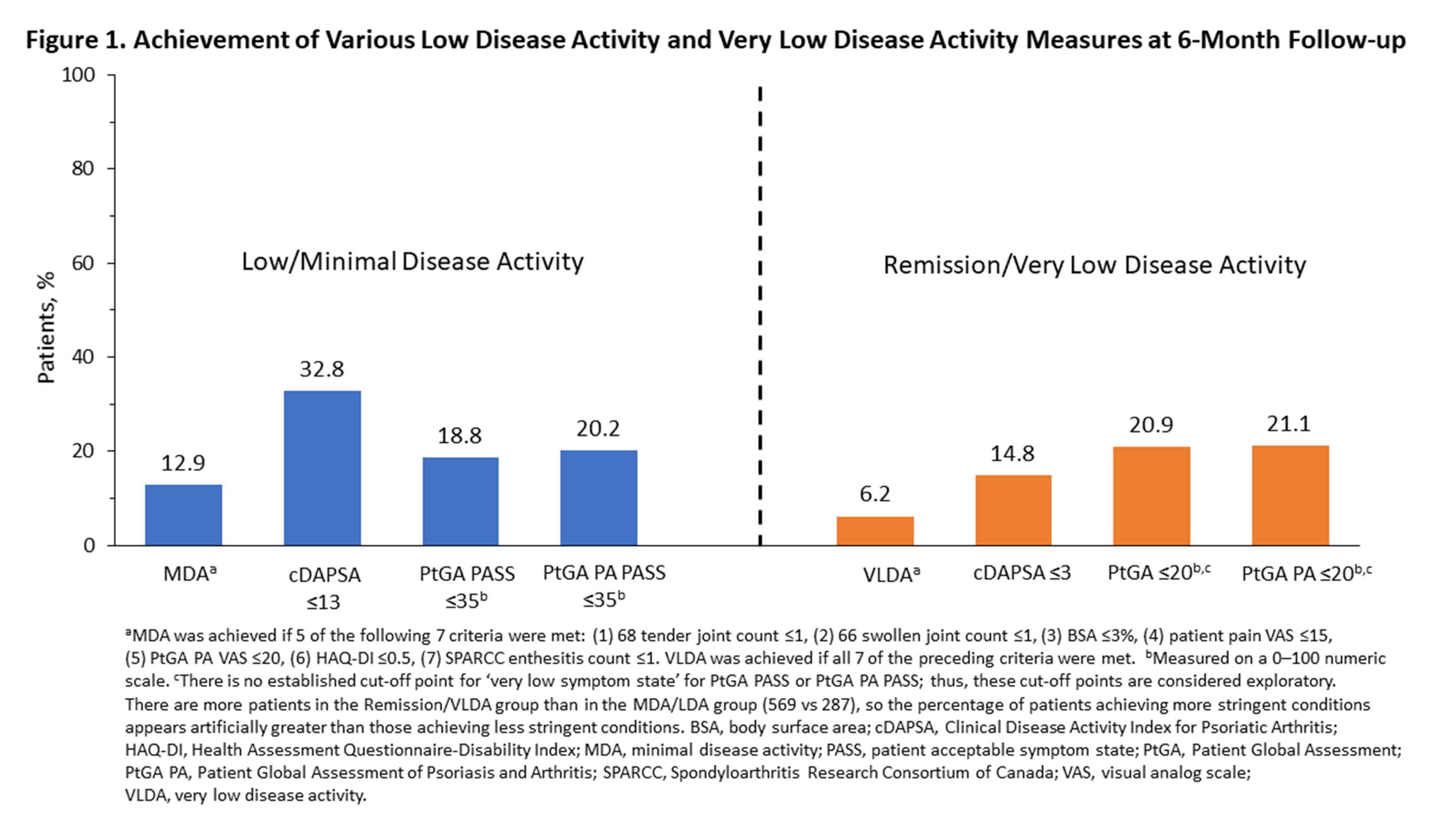
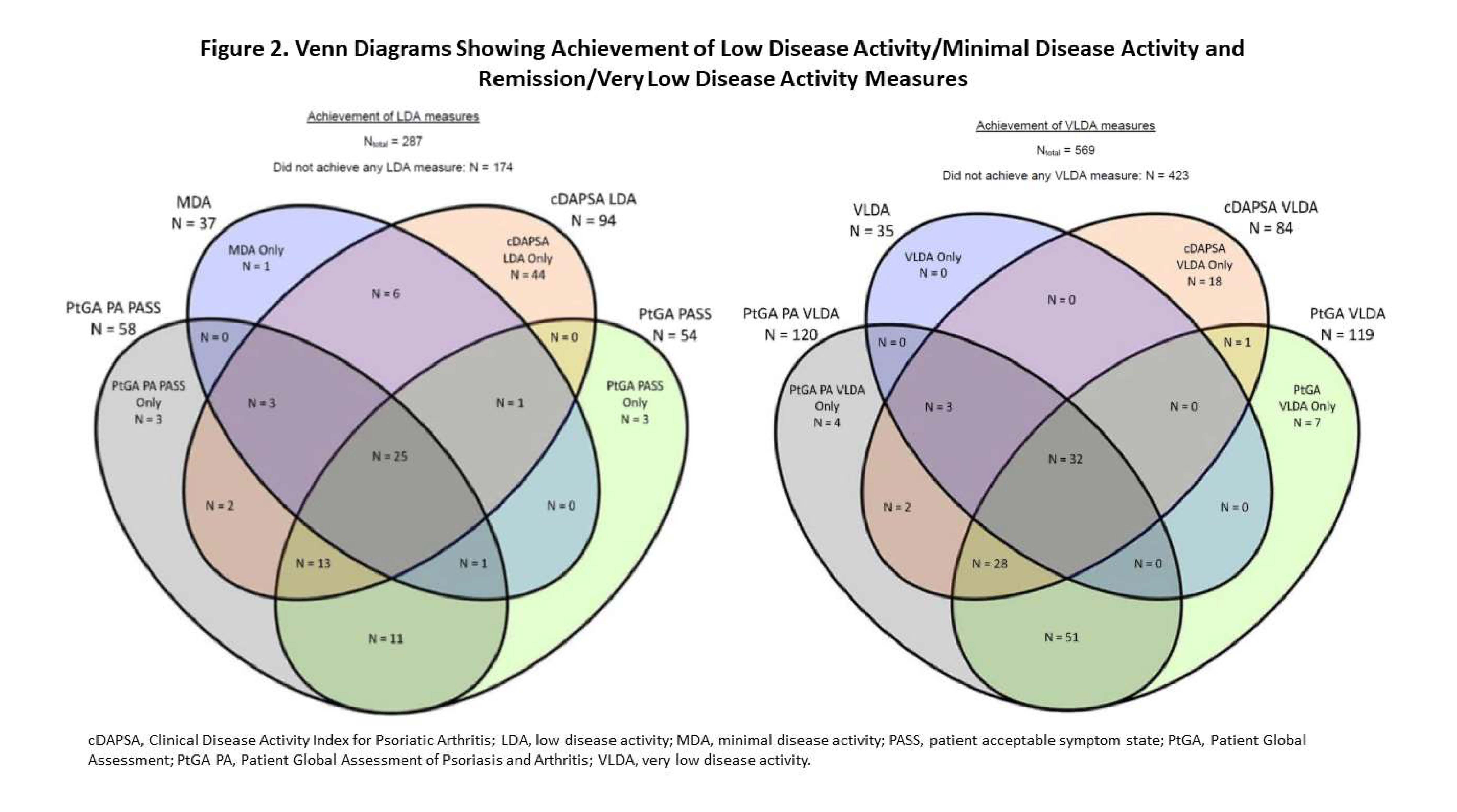
Results: A total of 287 and 569 patients were eligible for the LDA/MDA and remission/VLDA analyses, respectively. Most patients were taking a TNF inhibitor at baseline. The proportion of patients who achieved LDA/MDA at 6 months differed across the LDA/MDA measures (Figure 1). MDA had the lowest rate (12.9%) of achievement vs cDAPSA (32.8%) which had the highest rate. At 6 months, patients who achieved MDA reported less pain, lower tender joint, dactylitis, and enthesitis counts, less axial symptoms, and better physical function than those who met the cDAPSA, PtGA PASS, and PtGA PA PASS thresholds for LDA (Table 1). Percentages of patients who achieved remission/VLDA differed across remission/VLDA measures. VLDA had the lowest rate (6.2%) of achievement vs PtGA PA (21.1%) which had the highest rate at 6-month follow-up. Patients achieving VLDA reported less pain and axial involvement, and better physical function than patients achieving less stringent remission/VLDA thresholds for cDAPSA, PtGA PASS, and PtGA PA PASS (Table 1). Relationships among and between measures of PsA disease severity are illustrated in Figure 2.
Conclusion: MDA and VLDA were the most stringent disease activity measures of the respective criteria in patients with PsA and resulted in overall lower disease activity in all of the domains compared with patients who met cDAPSA, PtGA PASS, and PtGA PA PASS thresholds. Rheumatologists should be encouraged to use MDA and/or VLDA to assess disease control in their patients with PsA.
Medical writing services provided by Joann Hettasch of JK Associates, Inc. (a Fishawack Health Company) and funded by AbbVie.
To cite this abstract in AMA style:
Mease P, McLean R, Blachley T, Anatale-Tardiff L, Saffore C, Zueger P, Ogdie A. Comparison of Disease Control Thresholds in Psoriatic Arthritis: Results from the Corrona Psoriatic Arthritis/Spondyloarthritis Registry [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/comparison-of-disease-control-thresholds-in-psoriatic-arthritis-results-from-the-corrona-psoriatic-arthritis-spondyloarthritis-registry/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparison-of-disease-control-thresholds-in-psoriatic-arthritis-results-from-the-corrona-psoriatic-arthritis-spondyloarthritis-registry/