Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: We previously published a proof-of-principle study demonstrating the potential utility of computer vision (Deep Neural Network/DNN) methods applied to stained skin biopsy sections from patients with systemic sclerosis (SSc) as a novel skin outcome. The present study compared the ‘DNN-Fibrosis Score’ with histopathologic and modified Rodnan Skin Score (mRSS) changes for participants enrolled in a clinical trial.
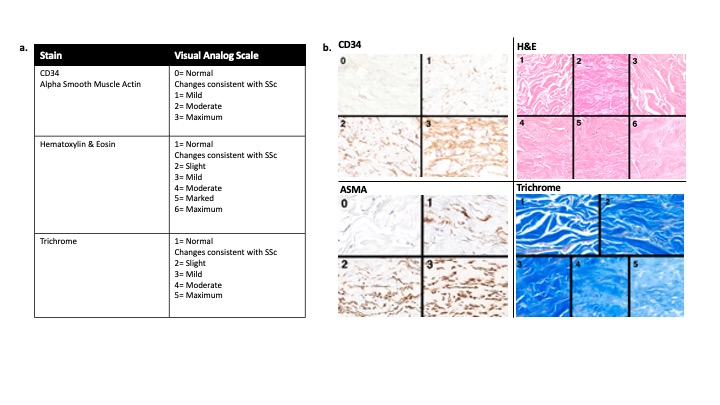
Methods: Ten adults with early (≤ 6 y) diffuse cutaneous SSc (15 ≤ mRSS ≤ 35) in an open-label belumosudil (ROCK2 inhibitor, 200 mg PO BID) trial had mRSS and two, 4mm, dorsal arm skin biopsies performed at weeks 0, 24, and 52. Biopsies were stained with CD34, CD3, CD8, alpha smooth muscle actin (ASMA), H&E, and trichrome. Two blinded dermatopathologists assessed biopsies for 16 histopathological parameters important in SSc (Van Praet JT et al., 2011). CD3+, CD8+ were counted, and visual analogue scales (VAS) were used to score CD34, ASMA, and relative SSc severity on H&E and trichrome (Fig. 1). A previously developed DNN algorithm (AlexNet) was applied to trichrome-stained images to generate a ‘DNN-Fibrosis Score’ as previously reported. ‘DNN-Fibrosis Scores’ were compared to mRSS using a linear regression model and Spearman correlation. Histopathologic parameters were compared to mRSS or DNN Fibrosis-Scores using logistic regression models. A p≤0.05 was considered significant.
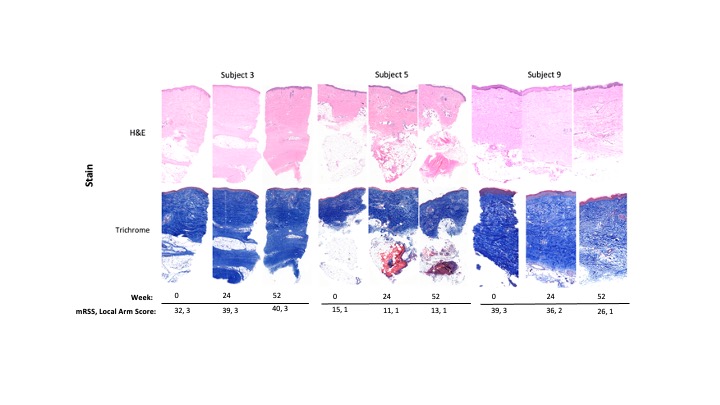
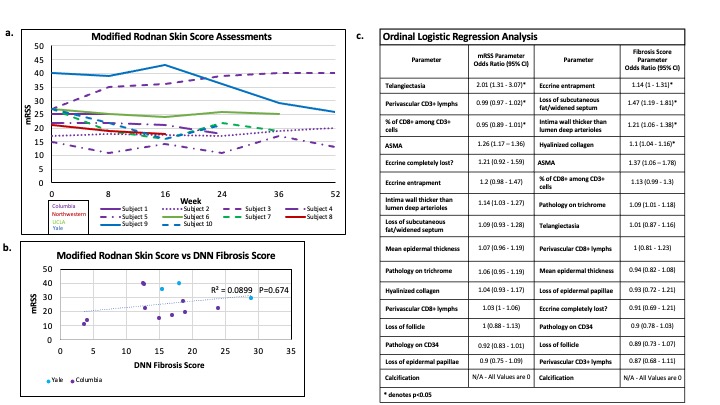
Results: Five patients had paired biopsies (Fig. 2). The median (interquartile range/IQR) mRSS change between 0 – 52W was -2.5 (-11 to 7.5) while the median (IQR) DNN-Fibrosis Score change (W0 – last follow-up) was -6 (-10.5 to 6.5) (Fig. 3). Of the histopathological scores, subcutaneous (SC) fat loss (p = 0.012), eccrine entrapment (p = 0.008), % CD8+ among CD3+ cells (p = 0.006) changed most during treatment. The correlation between mRSS and DNN-Fibrosis Score for the 5 paired biopsies was 0.18 [at higher mRSS, i.e. 25-51, the correlation was weaker] (Fig. 3b). Per 1-unit mRSS increase, the histopathological parameter odds ratios (OR); p-values were: telangiectasia =2.01; 0.001, perivascular CD3+ =1.03; 0.015, and % of CD8+ among CD3+ =1.08; 0.031 (Fig. 3c). Likewise, per 1-unit DNN Fibrosis-Score increase, OR; p-values for histopathological parameters were: hyalinized collagen =1.1; 0.00033, SC fat loss =1.47; 0.00033, intima wall =1.21; 0.005, and eccrine entrapment =1.14; 0.046 (Fig. 3c).
Conclusion: In this novel exploratory analysis, the DNN-Fibrosis Score exhibited sensitivity to histopathologic changes. The weak correlation between mRSS and DNN-Fibrosis Score contrasted with our previous findings. We note that predicted DNN-Fibrosis Scores tended to be lower for participants with higher mRSS ( >25). We attribute this divergence to batch effects from staining protocols between our published and our current analysis which may be overcome with analyses of larger cohorts. However, despite the weak correlation with mRSS, the DNN-Fibrosis Score significantly correlated with a set of histopathological variables that were distinct from those correlated with mRSS, including hyalinized collagen, SC fat loss, intima wall thickness, and eccrine entrapment.
To cite this abstract in AMA style:
Gunes B, Duran Camacho L, Cowper S, Panse G, Bundschuh E, Williams A, Page N, Karns M, Aren K, Pradhan N, Bernstein E, Fantus S, Volkmann E, Bukiri H, Correia C, Wilson F, Mawe S, Mahoney J, Hinchcliff M, Wang R. Comparing Deep Neural Network to Modified Rodnan Skin Score in a Trial for Belumosudil in Systemic Sclerosis Patients [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/comparing-deep-neural-network-to-modified-rodnan-skin-score-in-a-trial-for-belumosudil-in-systemic-sclerosis-patients/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparing-deep-neural-network-to-modified-rodnan-skin-score-in-a-trial-for-belumosudil-in-systemic-sclerosis-patients/