Session Information
Date: Monday, November 13, 2023
Title: (1100–1123) Metabolic & Crystal Arthropathies – Basic & Clinical Science Poster II
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: The management of gout remains poor despite the availability of effective medications and recommendation of a treat-to-target treatment strategy using of urate-lowering therapy (ULT). A serum urate level is required to make ULT dose adjustments. However, requiring lab work prior to a clinic visit can be burdensome for patients, while waiting for laboratory test results after the visit makes face-to-face counseling regarding any medication dose changes at the time of the visit impossible. Introducing a point-of-care (POC) device, much like a glucometer, would allow providers to perform serum urate testing during the clinic visit and make therapy adjustments as needed in real-time. Our purpose was to assess the accuracy, reliability, and acceptability of a POC device as compared to standard laboratory-based serum urate tests.
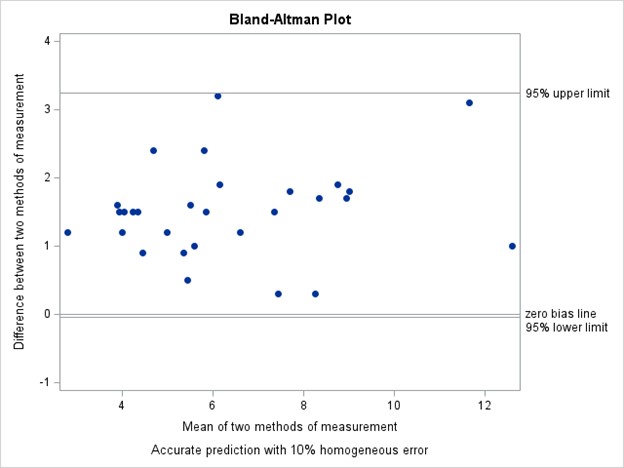
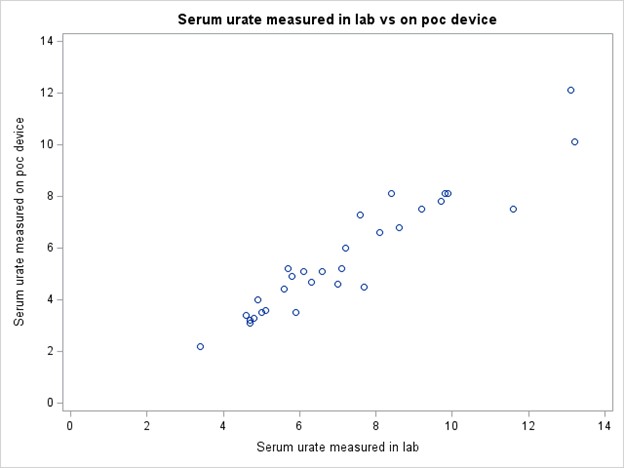
Methods: We measured serum urate levels in patients with gout using both a capillary POC device (Nova Max Uric Acid Monitoring System) that is FDA-approved for home use (but not approved for use in the clinic) and a standard laboratory-based test on the same day. A Bland-Altman analysis was conducted to identify whether the variability of the difference between tests was affected by the level of serum urate and to identify the limits of agreement between the two tests. We plotted the difference of the two values against the average value for each participant. Correlation between the two measurements of serum urate was evaluated using intraclass correlation coefficients with 95% confidence intervals (CI). In addition, we assessed the patient acceptability and convenience of device use through a self-reported standard Likert scale questionnaire.
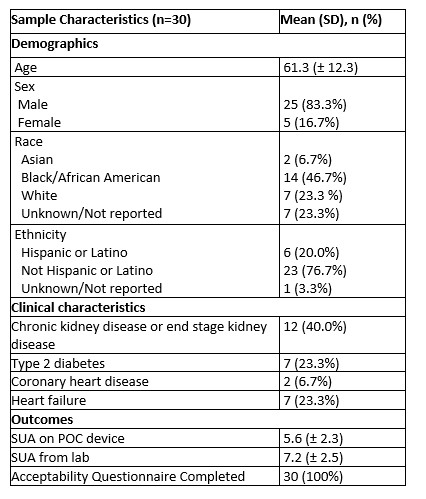
Results: We enrolled 30 patients with gout (mean age 61 ± 12.32, 83% male and 17% female; Table 1). The mean ± SD serum urate measured on the POC device was 5.65 ± 2.30 mg/dL and in the laboratory was 7.25 ± 2.52 mg/dL. The Bland-Altman plot (Figure 1) shows limits of agreement close to 0 and 3 indicating consistently lower measurements with the POC device. The intraclass correlation coefficient was 0.75 (95% CI 0.55 – 0.87), indicating moderate agreement (Figure 2). The results from the acceptability questionnaire suggest that the device was acceptable and liked by 97% of the participants. All the participants found it comfortable and 80% preferred the POC device over standard lab tests.
Conclusion: In this small pilot study, the POC device and the standard laboratory tests for serum urate were moderately correlated, though the POC device values were generally >1mg/dL lower than the laboratory values. This discrepancy in serum urate values indicates that this POC device is not suitable at the present time for making treatment decisions in the clinical setting for real-time dose titrations in patients with gout. Nonetheless, patients generally preferred a POC approach; thus, with improved accuracy, POC urate measurements could benefit real-time treat-to-target gout management.
To cite this abstract in AMA style:
Kehasse A, Dhamne S, LaValley M, Liew J, Neogi T. Comparing a Capillary Urate Point-Of-Care Device to Standard Laboratory-based Serum Urate Test for Dose Titration in Gout Patients [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/comparing-a-capillary-urate-point-of-care-device-to-standard-laboratory-based-serum-urate-test-for-dose-titration-in-gout-patients/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparing-a-capillary-urate-point-of-care-device-to-standard-laboratory-based-serum-urate-test-for-dose-titration-in-gout-patients/