Session Information
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
Background/Purpose: Since 2010, biological disease-modifying antirheumatic drugs (bDMARDs) have been the dominant mode of treatment for rheumatoid arthritis (RA). However, the safety of DMARDs, such as tumor necrosis factor inhibitors (TNFis) and Janus kinase inhibitors (JAKis), in treating patients with RA is a concern. We compared the safety outcomes of JAKis and TNFis in RA patients in clinical settings.
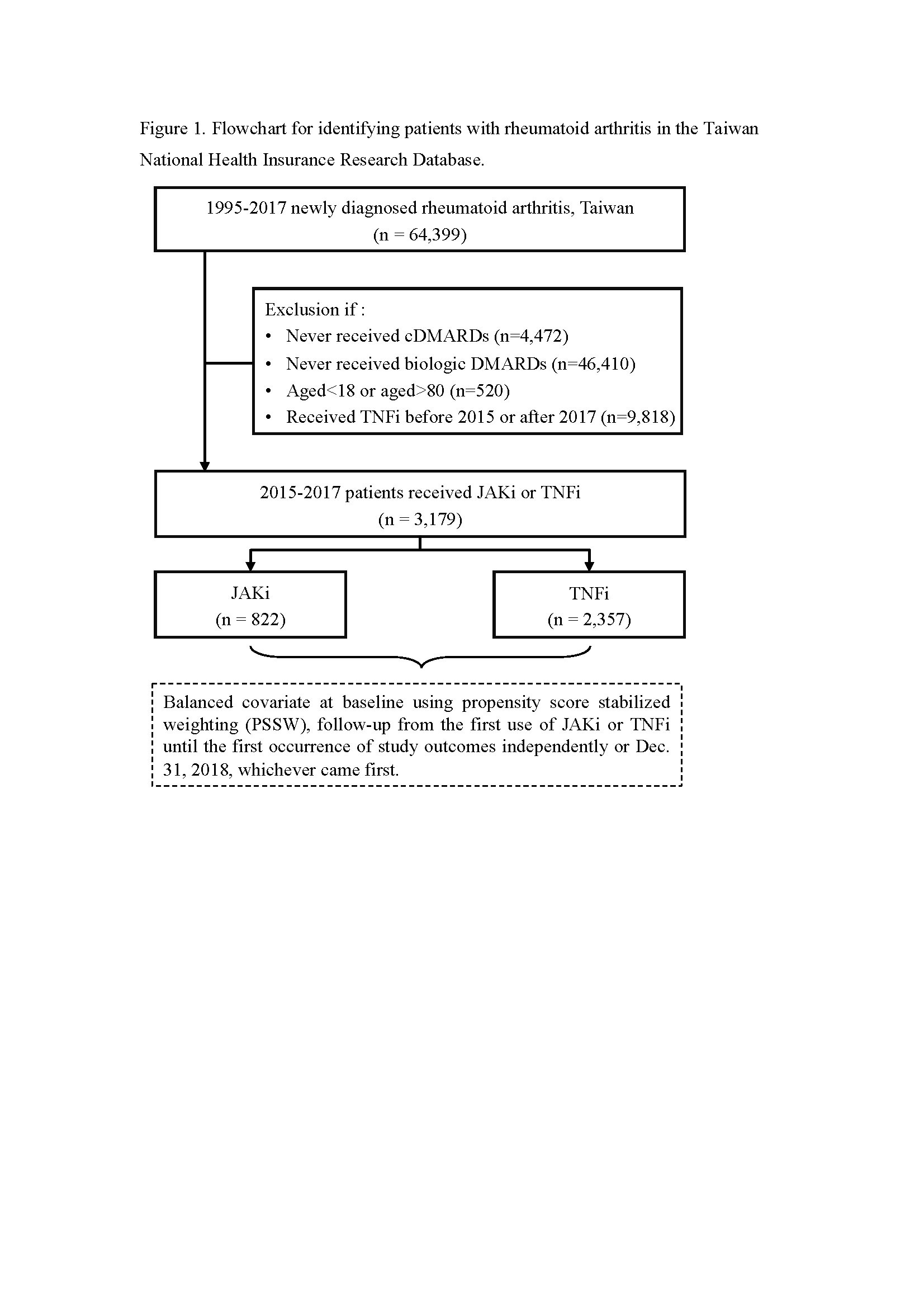
Methods: Patients diagnosed with RA between 2015 and 2017 were identified from the Taiwan National Health Insurance Research Database and followed till 2018. Propensity score stabilized weighting (PSSW) was used to balance the baseline characteristics of the JAKis and TNFis groups. The incidences of safety outcomes, namely cardiovascular (CV) events, tuberculosis (TB), total hip replacement (THR), total knee replacement (TKR), and all-cause mortality, were compared between the two study groups.
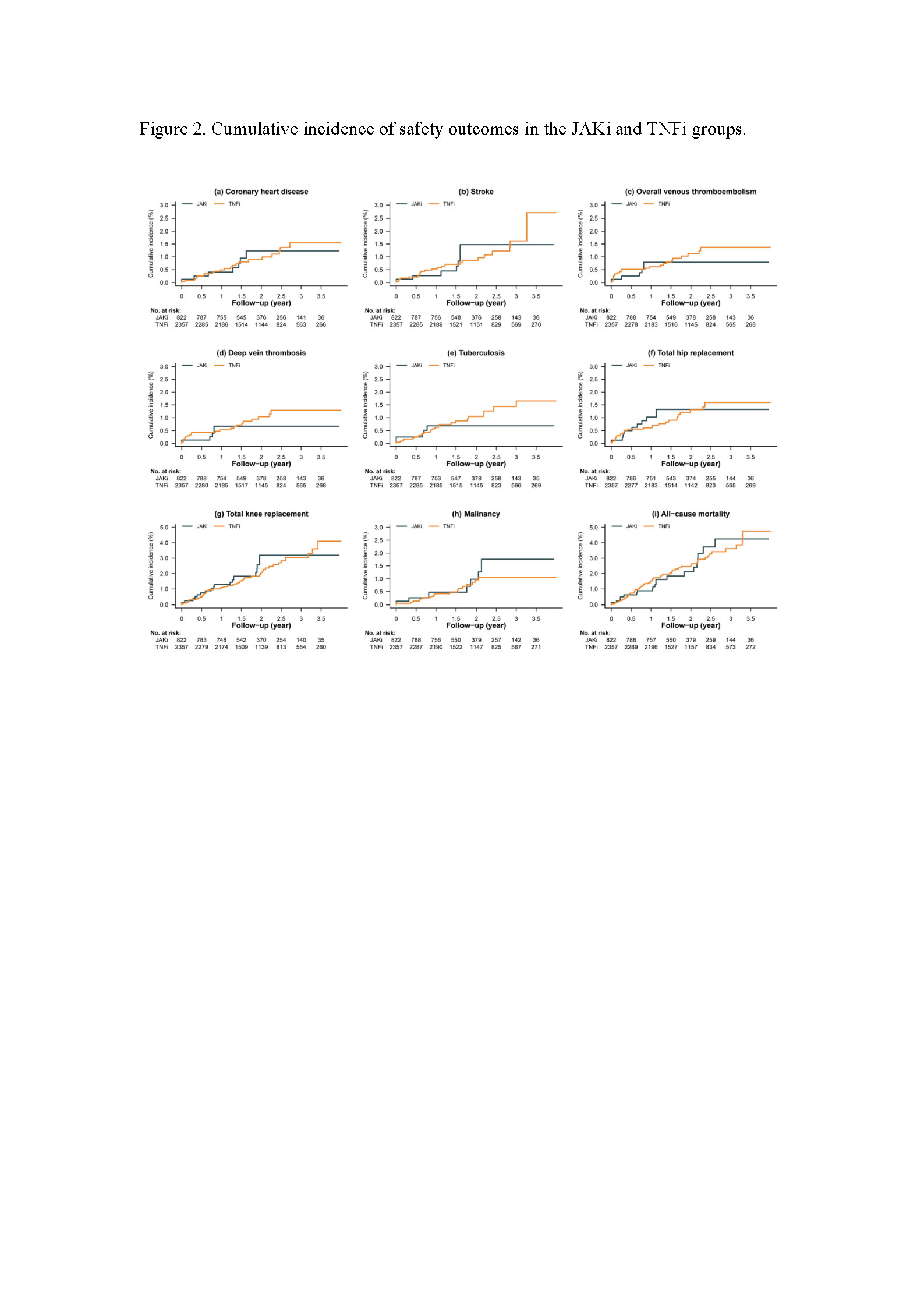
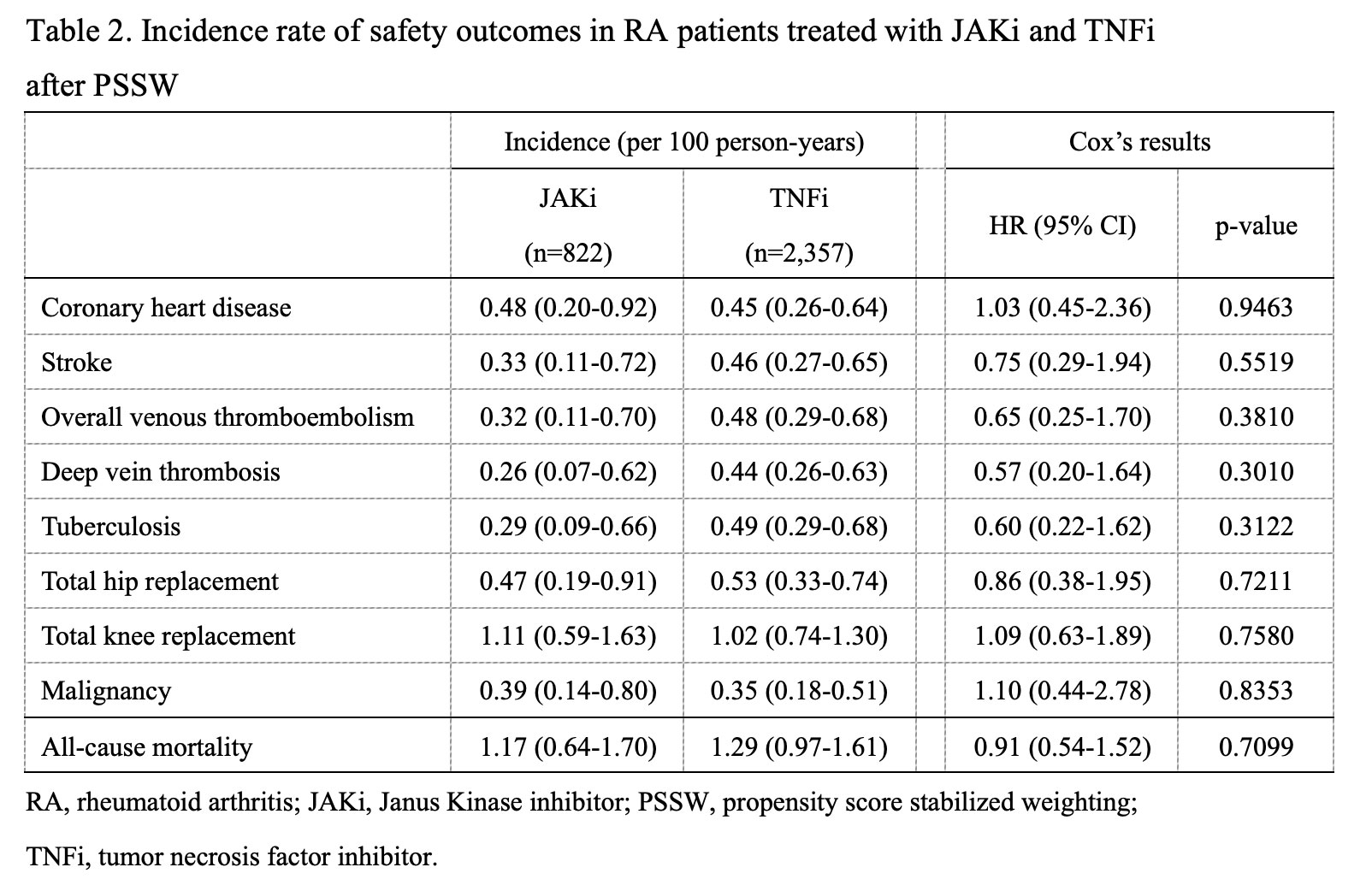
Results: A total of 3,179 patients with RA who were administered JAKis (n = 822) and TNFis (n = 2,357) were included in this study. The mean follow-up duration was 2.02 years in the JAKis group and 2.10 in the TNFis group. All-cause mortality had the highest incidence rate, followed by TKR, THR, CV events, and TB. A lower incidence rate of the study outcomes was observed in the JAKis group than in the TNFis group but without statistical significance.
Conclusion: Comparable safety issues and mortality rates were observed for JAKis and TNFis in RA patients treated in real-world settings.
To cite this abstract in AMA style:
Fang Y, See L. Comparative Safety of Janus Kinase Inhibitors and Tumor Necrosis Factor Inhibitors in Patients Undergoing Treatment for Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/comparative-safety-of-janus-kinase-inhibitors-and-tumor-necrosis-factor-inhibitors-in-patients-undergoing-treatment-for-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparative-safety-of-janus-kinase-inhibitors-and-tumor-necrosis-factor-inhibitors-in-patients-undergoing-treatment-for-rheumatoid-arthritis/