Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Current practice guidelines recommend either rituximab (RTX) or cyclophosphamide (CYC) for treatment of organ-threatening manifestations of systemic vasculitis or connective tissue disease (e.g., diffuse alveolar hemorrhage, active class III, IV, or V lupus nephritis). These diseases are associated with high mortality and are often refractory to standard therapies. Few studies have evaluated the safety and efficacy of combination therapy with RTX and CYC. We conducted this study to evaluate outcomes in the first 12 months following RTX-CYC combination therapy in pediatric patients with rheumatic diseases.
Methods: Patients who received combination RTX-CYC therapy for a rheumatic disease between January 2020 and February 2023 at a single center were included. The primary outcomes of interest were death and infection requiring hospitalization within 12 months of combination therapy. Secondary outcomes included change in serologic lupus disease activity markers and corticosteroid (CS) dose, flare in disease activity, infusion reactions, and incident hypogammaglobulinemia. Patient demographic information, outpatient medications, laboratory data, and admission data were collected for 12 months post-treatment.
Results: Eighty-nine pediatric patients received combination RTX-CYC therapy for a rheumatic disease during the study period. There were no reported deaths; eight patients (8.9%) were hospitalized with probable or definite infection. Patients with SLE demonstrated significant improvements across all serologic disease activity markers (anti-double stranded DNA antibodies, C3, C4) with p < 0.0001. The mean daily prednisone-equivalent dose significantly decreased by the end of the follow-up period from 54 mg ± 23.6 to 11 mg ± 9.3 (p< 0.0001), and 54 patients (62%) were able to discontinue CS. Six patients (6.7%) experienced flare of disease and eleven patients (12%) experienced infusion reactions – with 1 patient having a severe reaction to RTX necessitating discontinuation. Twenty-six patients (31%) experienced incident hypogammaglobinemia, with (69%) of cases recovering counts by 24 months.
Conclusion: Combination therapy with RTX and CYC can be safely administered to children with rheumatic diseases. Risk of serious adverse events, disease flare, and infusion reactions requiring cessation of therapy is uncommon, allowing for effective treatment with decreased CS burden. Prospective controlled trials comparing combination therapy to standard therapy are needed.
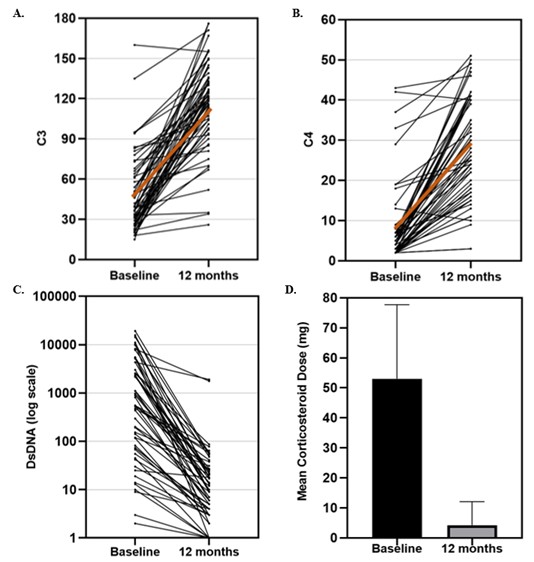 Figure 1. (A) Change in C3 levels in mg/dL – mean 47 ± 29 mg/dL prior to RTX-CYC and 115 ± 34 mg/dL at 12 months. (B) Change in C4 levels in mg/dL – mean 8 ± 10 mg/dL prior to RTX-CYC and 29 ± 13 mg/dL at 12 months. (C) Change in DsDNA levels in IU/mL – mean 2645 ± 4345 IU/mL prior to RTX-CYC and 85 ± 347 IU/mL at 12 months. The brown line represents the regression line. Improvements were significant at p < 0.0001. The mean daily prednisone-equivalent dose decreased from 54 mg ± 23.6 to 11 mg ± 9.3, p < 0.0001 by 12 months (D).
Figure 1. (A) Change in C3 levels in mg/dL – mean 47 ± 29 mg/dL prior to RTX-CYC and 115 ± 34 mg/dL at 12 months. (B) Change in C4 levels in mg/dL – mean 8 ± 10 mg/dL prior to RTX-CYC and 29 ± 13 mg/dL at 12 months. (C) Change in DsDNA levels in IU/mL – mean 2645 ± 4345 IU/mL prior to RTX-CYC and 85 ± 347 IU/mL at 12 months. The brown line represents the regression line. Improvements were significant at p < 0.0001. The mean daily prednisone-equivalent dose decreased from 54 mg ± 23.6 to 11 mg ± 9.3, p < 0.0001 by 12 months (D).
.jpg) Table 1: Demographics and Rheumatic Diseases, n=89
Table 1: Demographics and Rheumatic Diseases, n=89
.jpg) Table 2: Primary Indication for Rituximab and Cyclophosphamide Therapy, n=89
Table 2: Primary Indication for Rituximab and Cyclophosphamide Therapy, n=89
To cite this abstract in AMA style:
Rife E, Reiff D, Bridges J, Smitherman E, Cron r, Stoll M, Mannion M, Weiser P, Timmerman L. Combination Therapy with Rituximab and Cyclophosphamide for Treating Pediatric Patients with Severe Manifestations of Rheumatic Disease [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/combination-therapy-with-rituximab-and-cyclophosphamide-for-treating-pediatric-patients-with-severe-manifestations-of-rheumatic-disease/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/combination-therapy-with-rituximab-and-cyclophosphamide-for-treating-pediatric-patients-with-severe-manifestations-of-rheumatic-disease/
