Session Information
Date: Saturday, November 12, 2022
Title: RA – Animal Models Poster
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: Respiratory-related diseases are among the most common causes of death in rheumatoid arthritis (RA). Although autoimmune diseases are reported in higher frequency in females, lung disease of RA is disproportionately higher in men, which may be attributable to environmental and/or occupational risk factors. Prior animal studies demonstrated potentiated autoimmunity, arthritis, and pro-fibrotic/pro-inflammatory lung disease with the combination of airborne biohazard exposures and collagen-induced arthritis (CIA) (Poole et al. J Bone Min Res). These interactions were more predominant in male than female mice. The current study aimed to determine whether male hormone-dependent differences explained these observations.
Methods: Arthritis prone male normal (intact) and castrated DBA/1J mice (n=8/group x 2 experiments) received intranasal inhalation of lipopolysaccharide (LPS, 100 ng) daily for 5 weeks and CIA induction (collagen adjuvant injections on day 1 and day 21). At week 5, arthritis inflammatory scores, bronchoalveolar lavage fluid (BALF), lung tissues, and serum were collected for quantification of cellular influx, inflammatory/pro-fibrotic mediators, lung autoantigens, and autoantibody responses. Group differences were compared using Mann Whitney test.
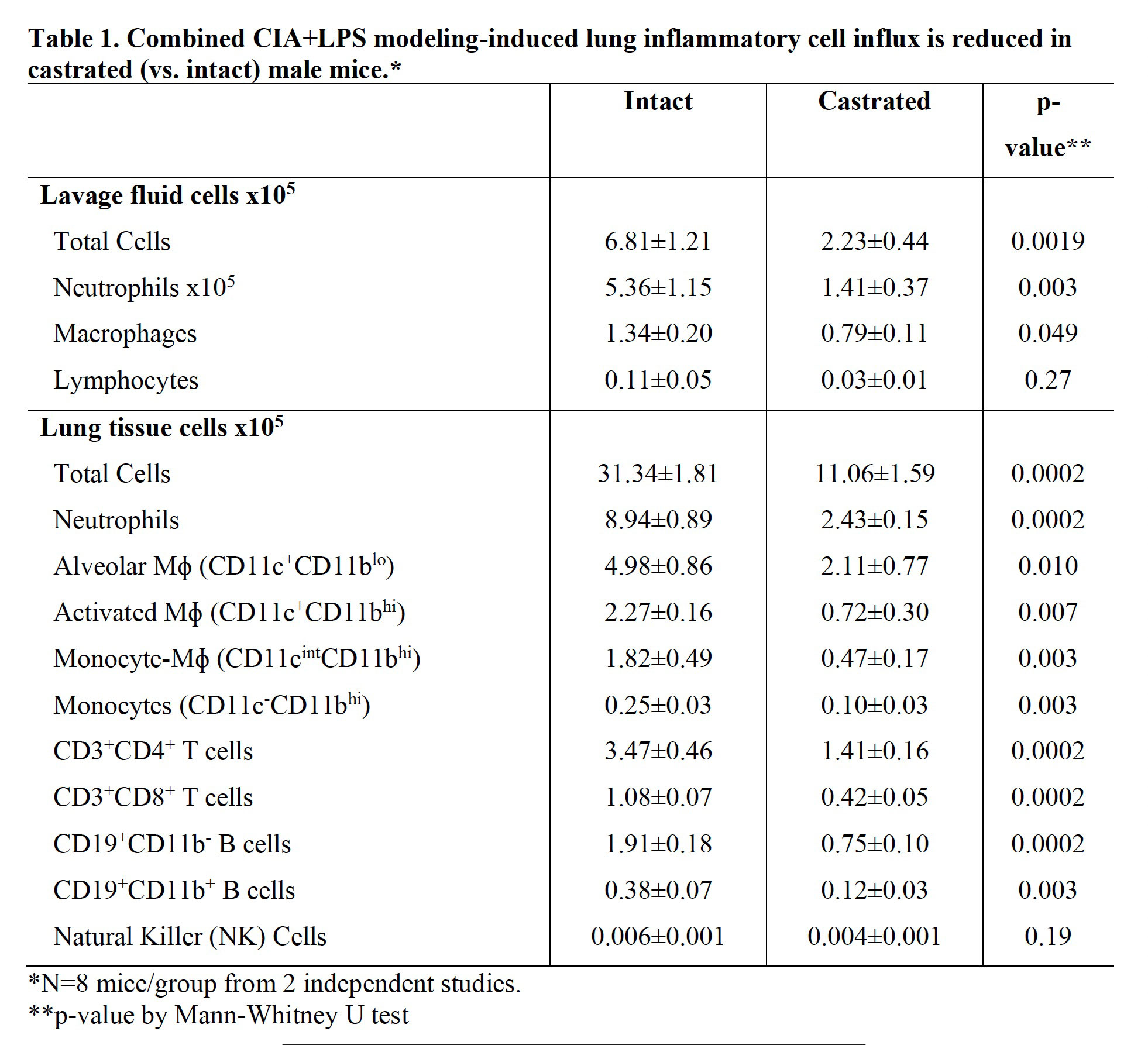
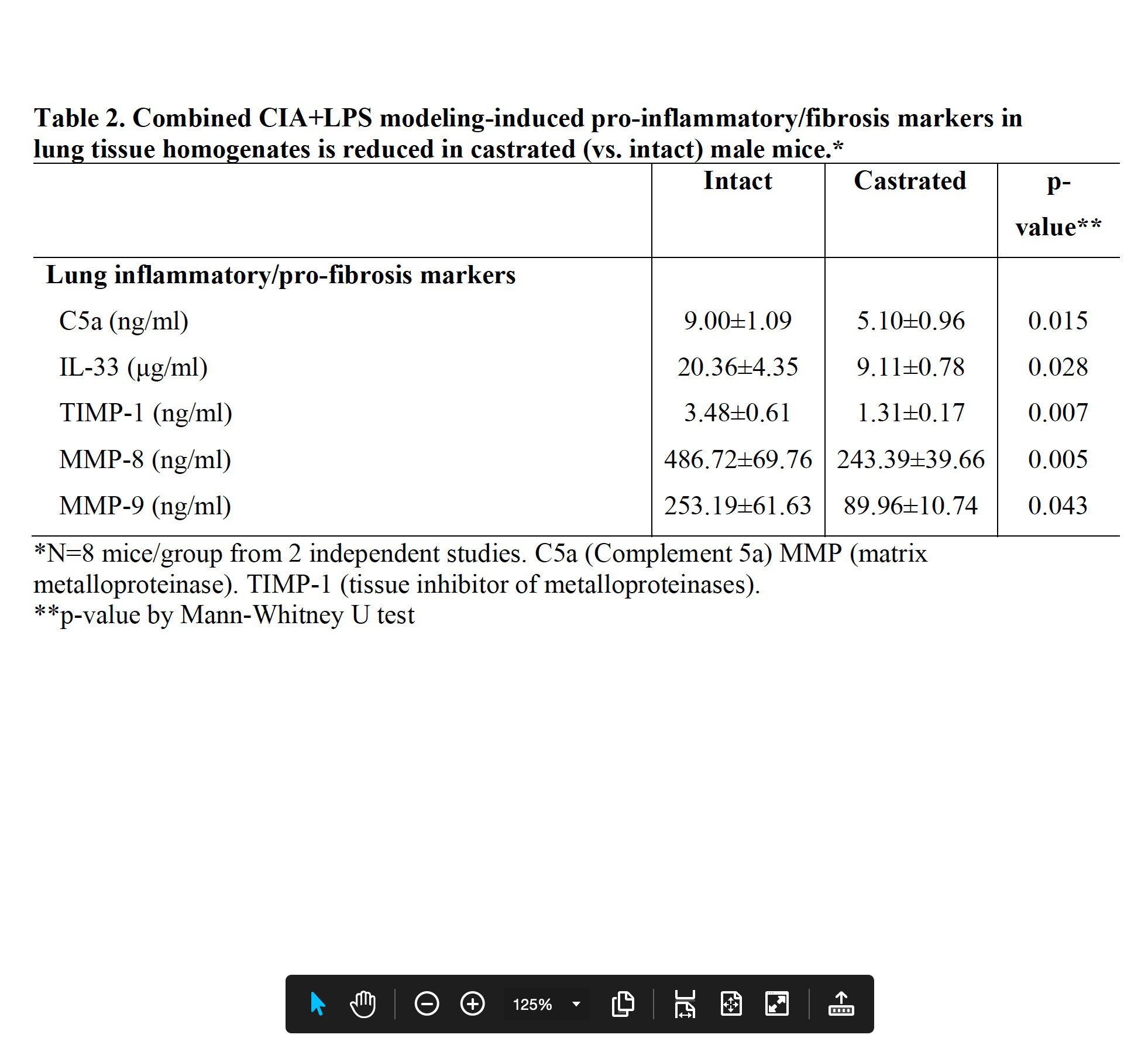
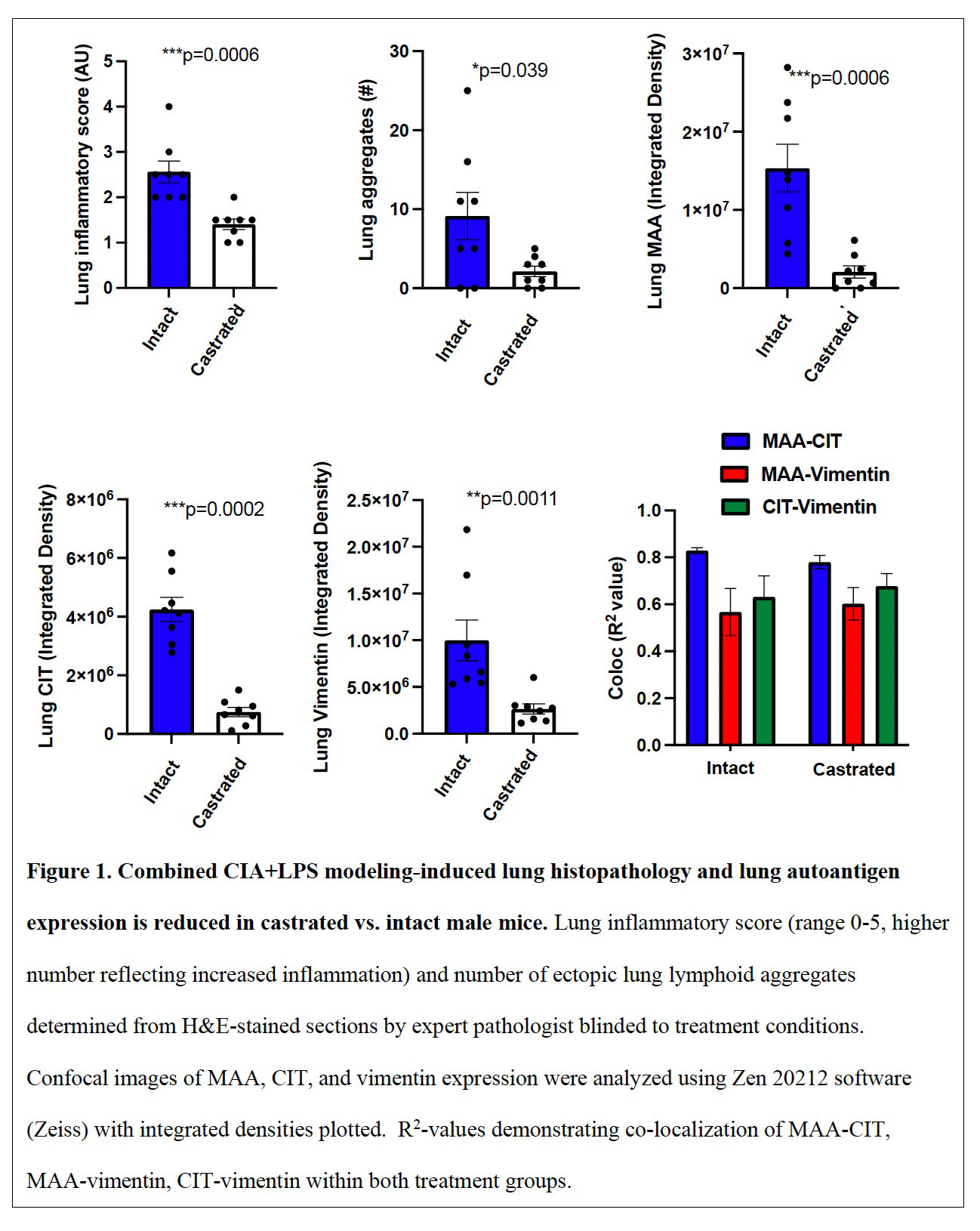
Results: Serum testosterone levels were increased in intact (10.2±2.3 ng/ml) vs. castrated (0.1±0.03) mice. At week 5, castrated mice had increased arthritis scores (mean±SEM) (1.8±0.4 vs. 0.8±0.1, p=0.03) and murine serum acute phase reactant pentraxin-2 levels (μg/ml; 233±105 vs. 52±4, p=0.02) vs. intact mice. However, CIA+LPS-induced BALF cell influx, lung tissue infiltrates (by flow cytometry), and lung homogenate levels of pro-inflammatory fibrotic markers including C5a, IL-33, and matrix metalloproteinases (MMPs) were reduced in castrated mice (Tables 1 and 2). Correspondingly, CIA+LPS-induced lung histopathology and expression of lung autoantigens including malondialdehyde acetaldehyde (MAA)- and citrulline (CIT)-modified lung proteins, and vimentin were reduced in castrated animals (Figure 1). There were no differences serum anti-MAA or ACPA levels between groups of mice induced with CIA + LPS.
Conclusion: CIA+LPS modeling in male mice profoundly induces influx of inflammatory cellular infiltrates, release of pro-inflammatory fibrotic mediators, and expression of lung autoantigens, all of which were strikingly reduced in castrated mice. This implies that testosterone is a major contributor to the lung inflammatory response to CIA+LPS co-exposure. In contrast, CIA+LPS-induced serum autoantibody responses were not dependent upon testosterone, and furthermore, arthritis and systemic inflammation were enhanced in the castrated mice. These observations demonstrate a compartmentalized effect of testosterone in this combined model and could serve to explain the epidemiologic and phenotypic differences in men and women with RA lung disease.
To cite this abstract in AMA style:
Ramler E, Mikuls T, Thiele G, Nelson A, Duryee M, Gaurav R, Wyatt T, Schwabb A, hunter c, England B, Poole J. Combination of Repetitive Inhalant Endotoxin Exposure and Collagen-induced Arthritis Interact in a Testosterone-dependent Manner to Drive Inflammatory Lung Disease Processes and Arthritis Severity in Mice [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/combination-of-repetitive-inhalant-endotoxin-exposure-and-collagen-induced-arthritis-interact-in-a-testosterone-dependent-manner-to-drive-inflammatory-lung-disease-processes-and-arthritis-severity-in/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/combination-of-repetitive-inhalant-endotoxin-exposure-and-collagen-induced-arthritis-interact-in-a-testosterone-dependent-manner-to-drive-inflammatory-lung-disease-processes-and-arthritis-severity-in/