Session Information
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Malondialdehyde-acetaldehyde adducts (MAA) are products of oxidative stress that modify self-proteins and stimulate potent cellular and humoral immune responses. We have previously demonstrated that MAA adducts are present in lung and joint tissues from rheumatoid arthritis (RA) patients and colocalize with citrullinated proteins. Moreover, anti-MAA antibodies are associated with higher RA disease activity and extra articular features including interstitial lung disease (ILD). However, the adducted protein(s) driving these immune responses are unknown. Implicated as pathogenic target autoantigens; we characterized extracellular matrix (ECM) protein and MAA expression in RA lung and joint tissues.
Methods: Paraffin embedded joint tissues from osteoarthritis (OA) and RA patients (n=5 each) were stained for MAA, vimentin, fibronectin, and collagen II. Similarly, we stained non-paired lung tissues from subjects with RA-ILD and controls (n=3 each). Tissues were assessed with confocal microscopy and staining patterns were quantified using Zen and image J software. Co-localization of ECM proteins with MAA was determined using the Fiji plugin, Coloc 2. Pearson’s correlation coefficients were calculated and compared between disease groups for lung and synovium.
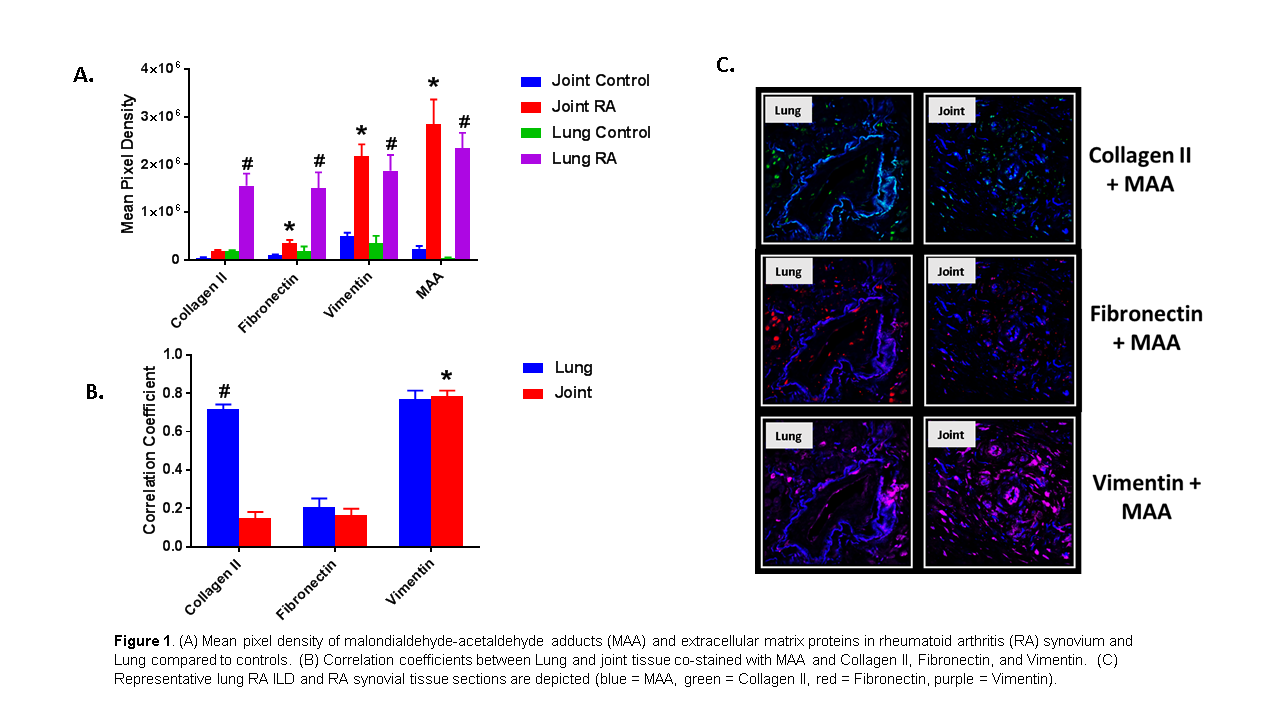
Results: MAA, vimentin, and fibronectin expression (but not collagen II) were all significantly higher in joint tissues from RA compared to OA patients (*P<0.001) Fig 1A. Colocalization of MAA with vimentin was significantly greater than that observed with other ECM proteins in RA synovium (*P<0.001) Fig 1B/C. In lung tissues, all 3 ECM proteins and MAA were all significantly increased in RA-ILD vs. normal controls (#P<0.03) Fig 1A. Colocalization of MAA with the ECM proteins (Collagen II and Vimentin) was increased in RA-ILD lung tissue. Similar to RA joint tissues, colocalization with MAA and vimentin was observed (R=0.77). However, collagen II was significantly increased (#P<0.001) in the lung compared to the joint Fig 1B/C.
Conclusion: Enhanced colocalization of critically important ECM proteins with MAA in diseased tissues, in addition to prior observations of increased anti-MAA antibody responses in RA, suggests a mechanistic role of MAA in RA synovial and lung disease. Moreover, these results suggest that interactions between MAA and ECM proteins are tissue dependent with MAA modification of vimentin potentially shared between the lung and synovium, while MAA modification of collagen may be more lung predominant.
To cite this abstract in AMA style:
Thiele GM, Duryee MJ, McGowan JD, Duryee LM, Klassen LW, O'Dell JR, England BR, Mikuls TR. Colocalization of Malondialdehyde-Acetaldehyde Adducts (MAA) and Extracellular Matrix Proteins in Joint and Lung Tissues from Rheumatoid Arthritis Patients [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/colocalization-of-malondialdehyde-acetaldehyde-adducts-maa-and-extracellular-matrix-proteins-in-joint-and-lung-tissues-from-rheumatoid-arthritis-patients/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/colocalization-of-malondialdehyde-acetaldehyde-adducts-maa-and-extracellular-matrix-proteins-in-joint-and-lung-tissues-from-rheumatoid-arthritis-patients/