Session Information
Date: Tuesday, November 14, 2023
Title: (2095–2140) RA – Diagnosis, Manifestations, and Outcomes Poster III
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Autoimmune rheumatic diseases (AIRDs) are systemic but heterogeneous diseases characterized by orchestration of disrupted self-tolerance of immune systems. We face a challenge in characterizing clinical immune features and selecting best treatment strategy of the AIRD patients. Deconvolution of immunological and clinical heterogeneity within and across AIRDs is an essential step towards implementation of personalized medicine. Immuno-phenotypes, immune-related cell types in peripheral blood mononuclear cells quantified by flow-cytometry analysis, can less invasively describe immune profiles of individuals and is considered as a promising technique.
Methods: We conducted large-scale and cohort-wide immuno-phenotyping of 46 peripheral immune cells using the Human Immunology Protocol of comprehensive 8-color flow cytometric analysis. The dataset consisted of >1,000 Japanese patients of 11 AIRDs and controls with deep clinical information registered at the FLOW study, including rheumatoid arthritis (RA), systemic lupus erythematosus (SLE),systemic sclerosis (SSc), ANCA related vasculitis (AAV), idiopathic inflammatory myopathy (IIM), psoriasis, IgG4 related disease (IgG4RD), mixed connective tissue disease (MCTD), ankylosing spondylitis (AS), Sjogren’s syndrome (SjS), and giant cell aortitis (GCA). In depth and longitudinal clinical analysis was conducted for the identified RA patient clusters registered at the FIRST registry. Inborn human genetics represented by genome-wide polygenic risk score (PRS) were estimated for the RA patients.
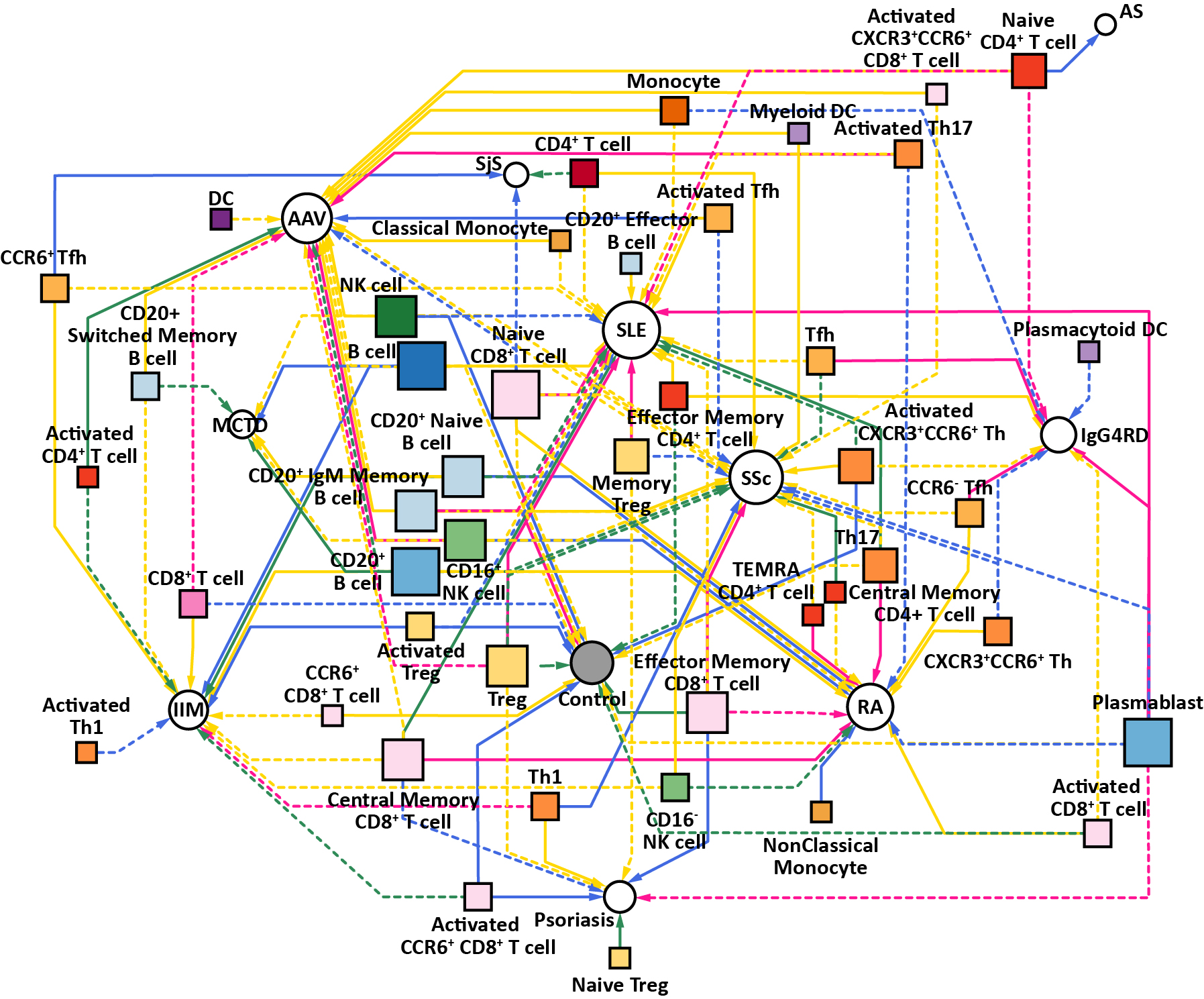
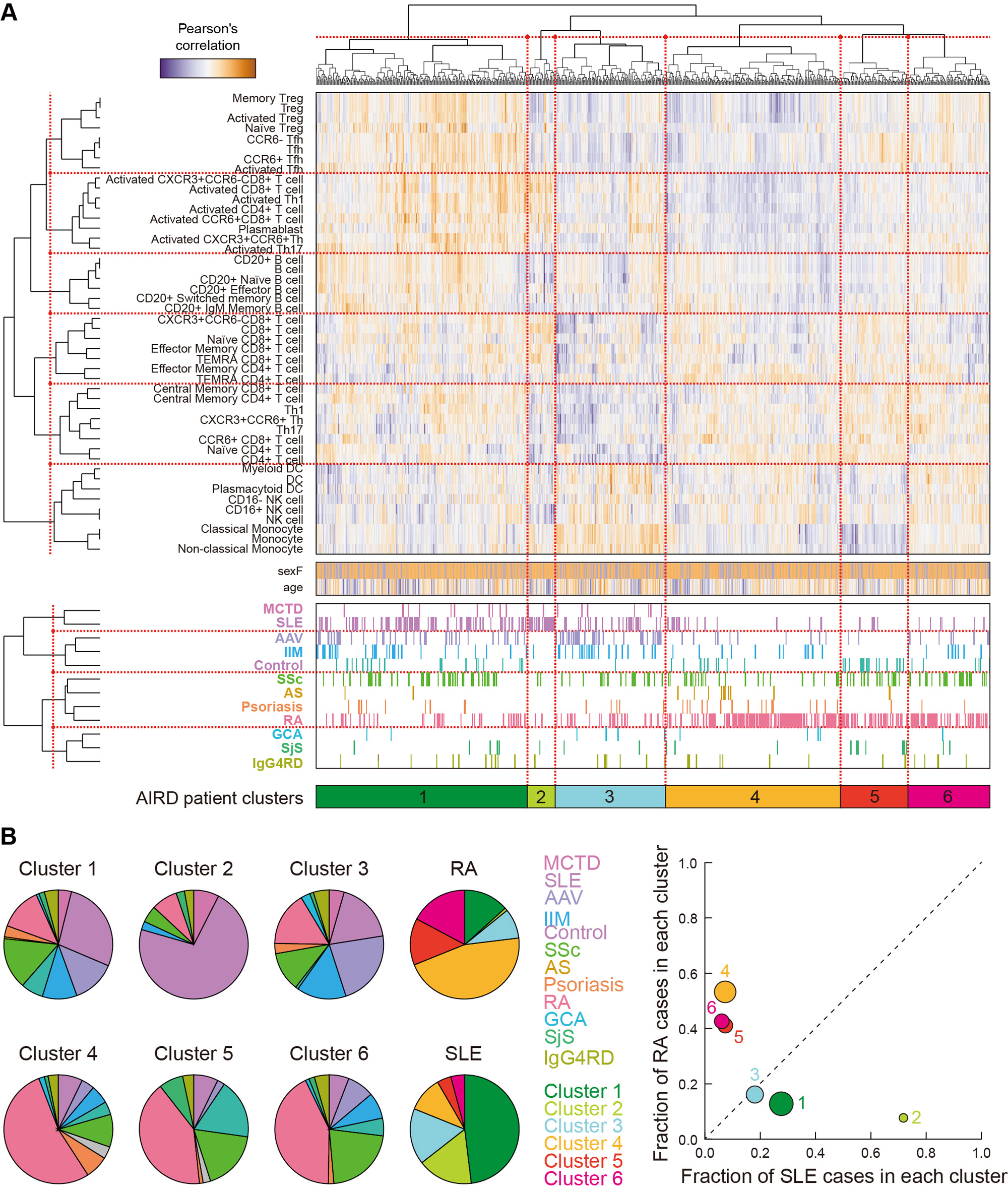
Results: Multimodal clustering of immuno-phenotypes deciphered underlying disease-cell type network, providing immune cell type specificity shared or distinct across AIRDs (Figure 1), such as close immunological network of MCTD with SLE rather than with SSc. Individual patient-level clustering deconvoluted the AIRD patients into several clusters with different immunological features (Figure 2). Of these, RA- or SLE-like clusters were exclusively dominant, showing immunological polarization between RA and SLE across AIRDs (the patient clusters 4-6 and 1-3 for RA and SLE, respectively). In depth and longitudinal clinical analysis of RA revealed that such patient clusters differentially defined clinical heterogeneity in disease activity and treatment responses, which were supported by immune cell-type specificity (e.g., decreased regulatory T cells associated with treatment resistance in the RA patients with SLE-like immuno-phenotypes). PRS based on RA case-control genome-wide association study (GWAS) and within-case stratified RA GWAS were associated with patient characteristics, disease activity, and immuno-phenotypes of the RA patients, such as dendritic cells for RA-interstitial lung disease (Figure 3).
Conclusion: Our study demonstrated a value of cohort-wide and cross-disease immuno-phenotyping to elucidate clinically heterogenous patient subtypes existing within the single disease in an immune cell type-specific manner.
AIRD-immune cell type associations based on backward-forward stepwise logistic regression adjusted by sex were visualized as a network plot.
(A) Unsupervised clustering of individual AIRD patients according to the immuno-phenotypes of the 46 peripheral immune cells. (B) Pie charts showing proportions of the AIRDs within each cluster, and those of the clusters within RA and SLE (left). A co-plot of the fractions of the RA and SLE patients within each cluster (right).
Associations of the PRS based on RA case-control GWAS and within RA case GWAS for RA-ILD with clinical information and immune-phenotypes of the cell types are indicated.
To cite this abstract in AMA style:
Tanaka H, Okada Y, Nakayamada S, Miyazaki Y, Sonehara K, Namba S, Honda S, Shirai Y, Yamamoto K, Ikari K, Harigai M, SONOMOTO K, Tanaka Y. Cohort-wide Immuno-phenotype Deconvolute Immunological and Clinical Heterogeneity Across Autoimmune Rheumatic Diseases [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/cohort-wide-immuno-phenotype-deconvolute-immunological-and-clinical-heterogeneity-across-autoimmune-rheumatic-diseases/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cohort-wide-immuno-phenotype-deconvolute-immunological-and-clinical-heterogeneity-across-autoimmune-rheumatic-diseases/