Session Information
Date: Monday, November 18, 2024
Title: T Cell Biology & Targets in Autoimmune & Inflammatory Disease Poster
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Anti-citrullinated protein antibody (ACPA) is highly specific to rheumatoid arthritis (RA). Beyond citrullination, other post-translational protein modifications including malondialdehyde-acetaldehyde (MAA) are targeted by T cells and autoantibodies in RA. MAA is abundant in RA synovial tissues and strongly co-localizes with both citrulline and B cells, suggesting that MAA expression could modulate adaptive immune responses to citrullinated proteins. In this study, we quantified changes T cell responses and autoantibody reactivity resulting from co-modification of a citrullinated protein with MAA.
Methods: Peripheral blood mononuclear cells (PBMCs; n=10) and serum (n=2484) were collected from RA participants in the Nebraska Rheumatology Biobank (NRB) and Veterans Affairs Rheumatoid Arthritis Registry (VARA), respectively. PBMCs (NRB) were isolated and stimulated with citrullinated fibrinogen (FIB-CIT), MAA-modified FIB (FIB-MAA), or co-modified FIB (FIB-MAA-CIT) for 48 hrs, followed by incubation with autologous T cells and evaluated for: 1) T cell proliferation using IFN-γ ELISpot kits; or 2) Th1, Th2, T-Reg, and Th17 cell types by flow cytometry. ELISpot and flow cytometry data were analyzed using one-way ANOVA with Tukey’s multiple comparison test. Serum levels (relative units) of IgG antibody to FIB-CIT, FIB-MAA, and FIB-MAA-CIT were quantified via ELISA (VARA). Antibody positivity was established as >2 SD above mean levels quantified in a separate cohort of 103 healthy controls. Seropositivity to modified and co-modified fibrinogen was compared by chi-square tests.
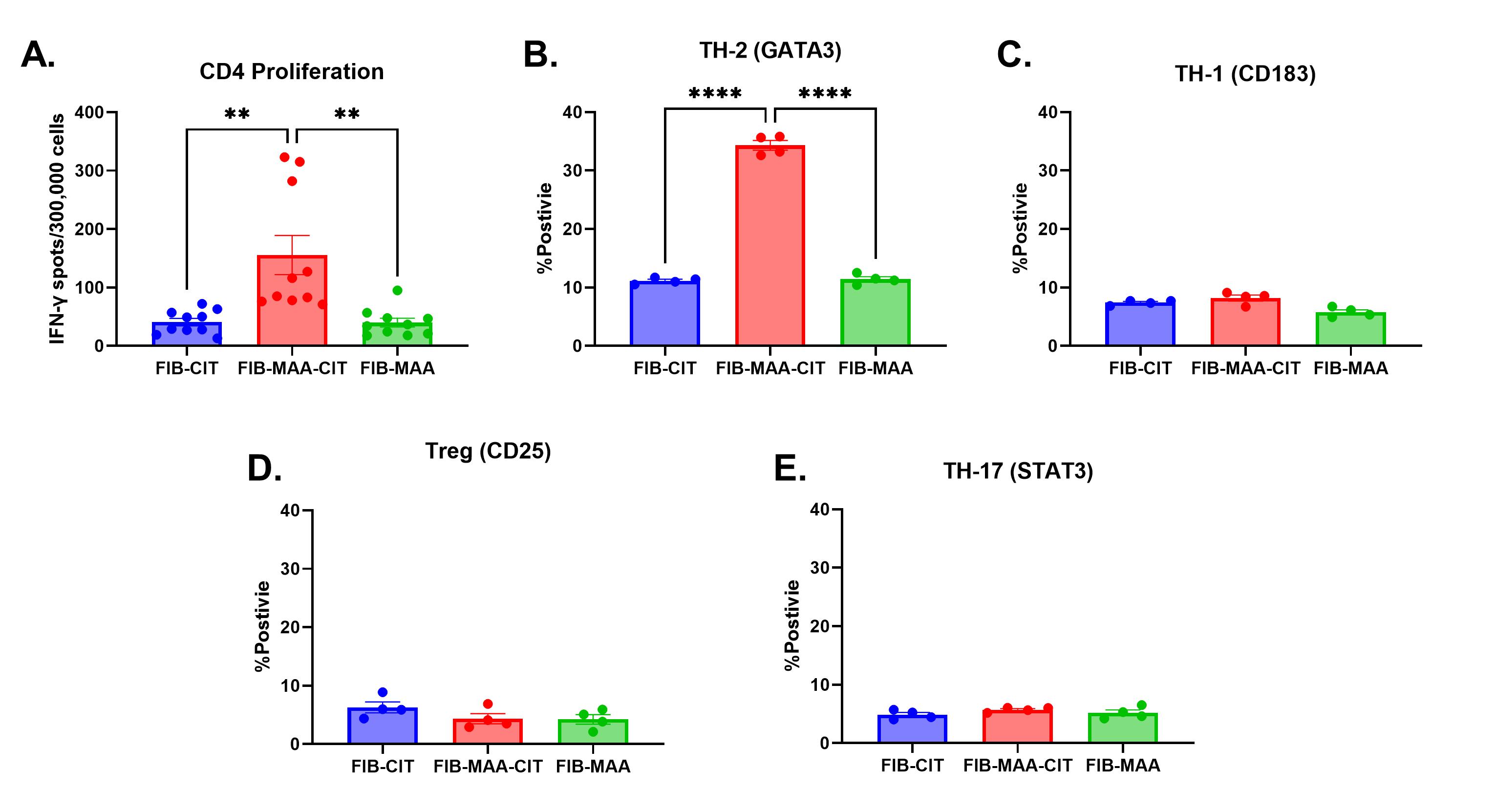
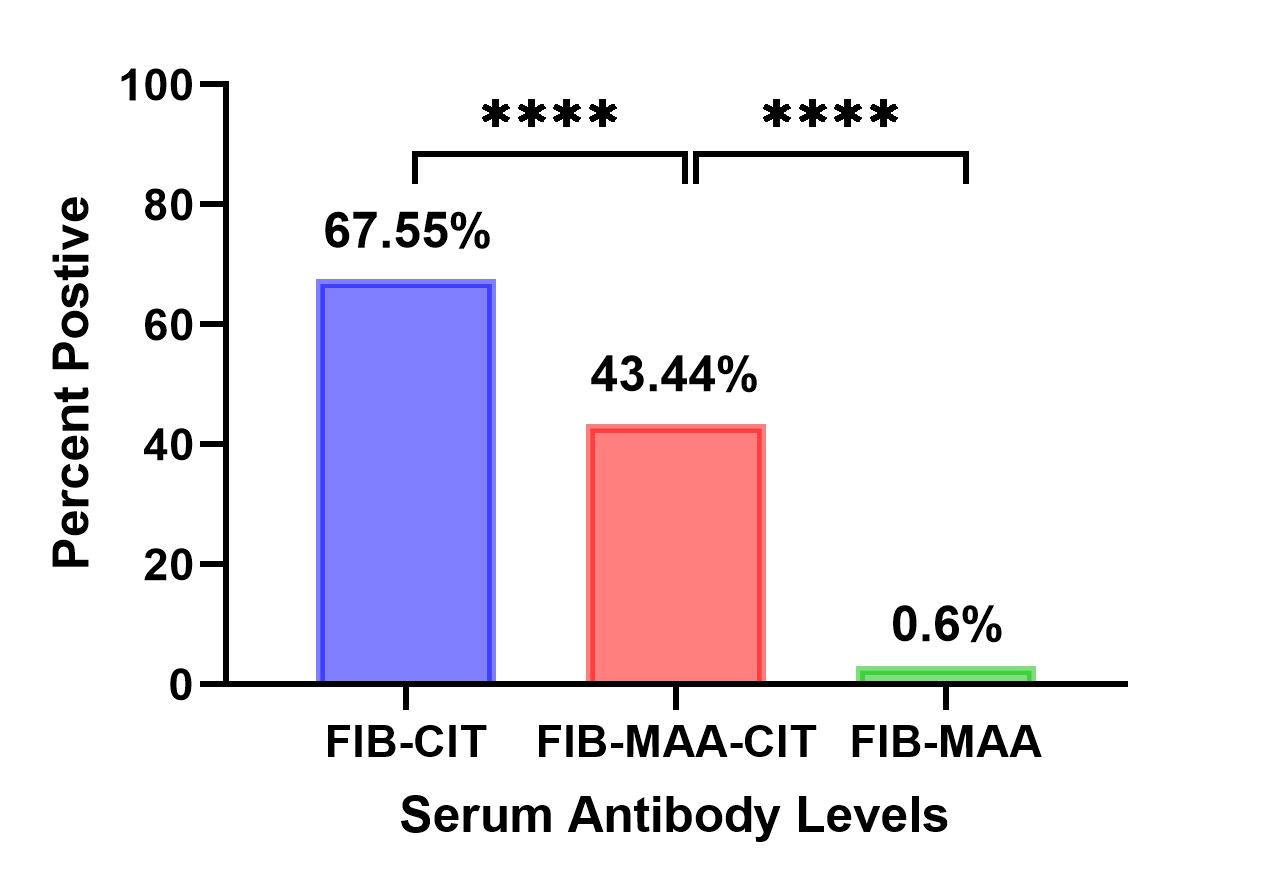
Results: Participant characteristics were similar in the 2 cohorts (90-91% male; mean age 63-73 years; 78-100% anti-CCP positive). CD4 cells proliferated significantly more to FIB-MAA-CIT than either FIB-CIT or FIB-MAA (p< 0.01) (Fig. 1A). This proliferation represented a predominant Th2 response based on increased GATA3 %-positivity in response to FIB-MAA-CIT vs. FIB-CIT or FIB-MAA (p< 0.0001) (Fig. 1B). In contrast, Th1 (CD183) (Fig. 1C), T-Reg (CD25) (Fig.1D) and Th17 (STAT3) %-positivity (Fig. 1E) showed no differences between FIB-CIT, FIB-MAA, and FIB-MAA-CIT. In VARA, the proportion of participants positive for serum IgG antibodies to FIB-CIT (68%) was significantly greater than positivity for either FIB-MAA-CIT (43%) or FIB-MAA (0.6%) (p˂0.0001) (Fig. 2).
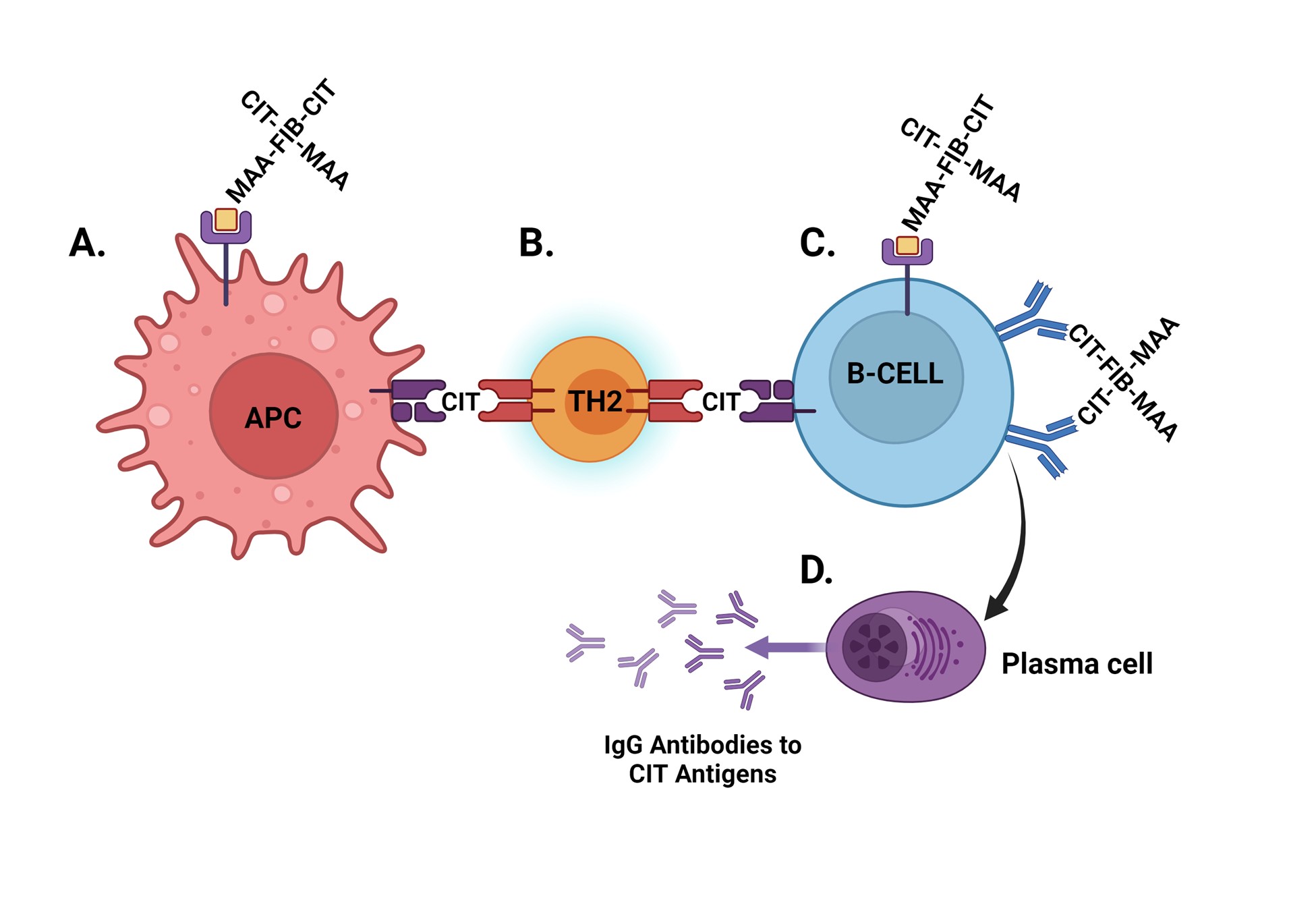
Conclusion: These results demonstrate that co-modification of CIT-protein with MAA significantly amplifies CD4 T cell proliferation and promotes a Th2 response to antigen stimulation. Given the known role of Th2 cells in promoting autoantibody expression, our data further suggest that resulting T cell responses promote the generation of ACPA but not anti-MAA antibody. Figure 3 provides a summary of the hypothesis for the mechanism behind these results. Additional investigation is needed to understand whether these observations are driven by linked recognition (binding and uptake of co-modified antigen followed by preferential processing and presentation of CIT antigen) and whether interventions targeting MAA could reduce pathologic immune responses to CIT-protein in RA.
To cite this abstract in AMA style:
Butler B, Zhou W, Duryee M, Aripova N, Sharp E, Hunter C, Kramer B, Sayles H, O'Dell J, Thiele G, England B, Baker J, Reimold A, Kerr G, Mikuls T. Co-modification of Citrullinated Proteins with Malondialdehyde-Acetaldehyde Leads to Amplified T Cell Responses and Increased Disease-specific Autoantibody Concentrations [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/co-modification-of-citrullinated-proteins-with-malondialdehyde-acetaldehyde-leads-to-amplified-t-cell-responses-and-increased-disease-specific-autoantibody-concentrations/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/co-modification-of-citrullinated-proteins-with-malondialdehyde-acetaldehyde-leads-to-amplified-t-cell-responses-and-increased-disease-specific-autoantibody-concentrations/