Session Information
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Inflammatory cytokines such as TNF-α, IL-6, and IFN-γ show their peaks at midnight in sera of patients with rheumatoid arthritis (RA), which is thought to be related to “morning stiffness of joints” as circadian manifestations. Circadian rhythms in individual cells are regulated by the expressions of clock genes, and we have previously reported that clock gene Bmal1 promotes production of inflammatory mediators in RA fibroblast-like synovial cells (RA-FLS) (Kaneshiro. K et al. BBRC, 2024, 691:149315). NF-κB, an important transcription factors for RA, plays a role in various inflammatory responses such as an interaction between BMAL1 and MMP-9 in breast cancer cells (Wang et al. Cancer Cell Int, 2019, 19:182). In this study, we examined how Bmal1 contributes to the production of inflammatory mediators in RA-FLS in terms of NF-κB.
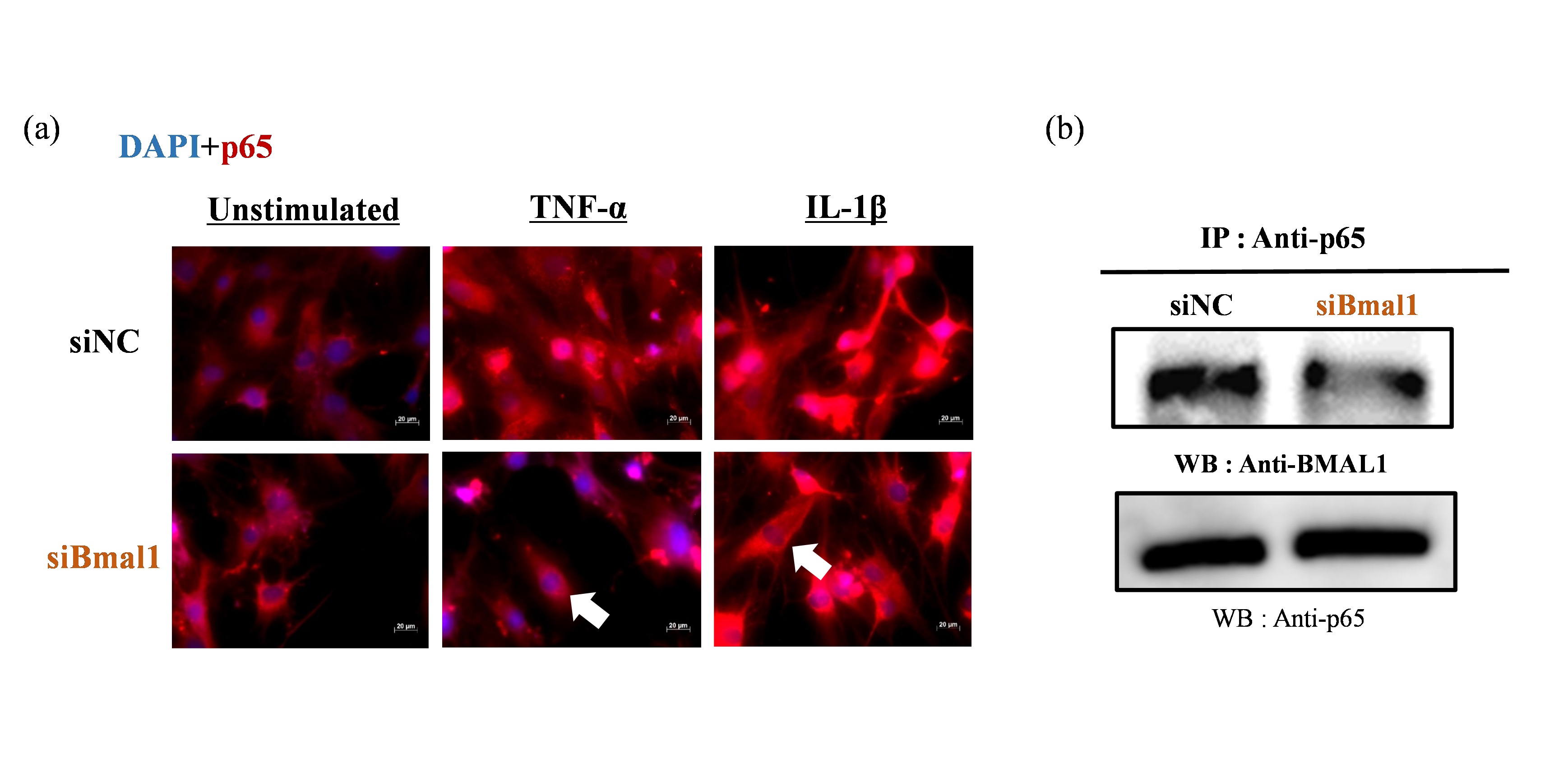
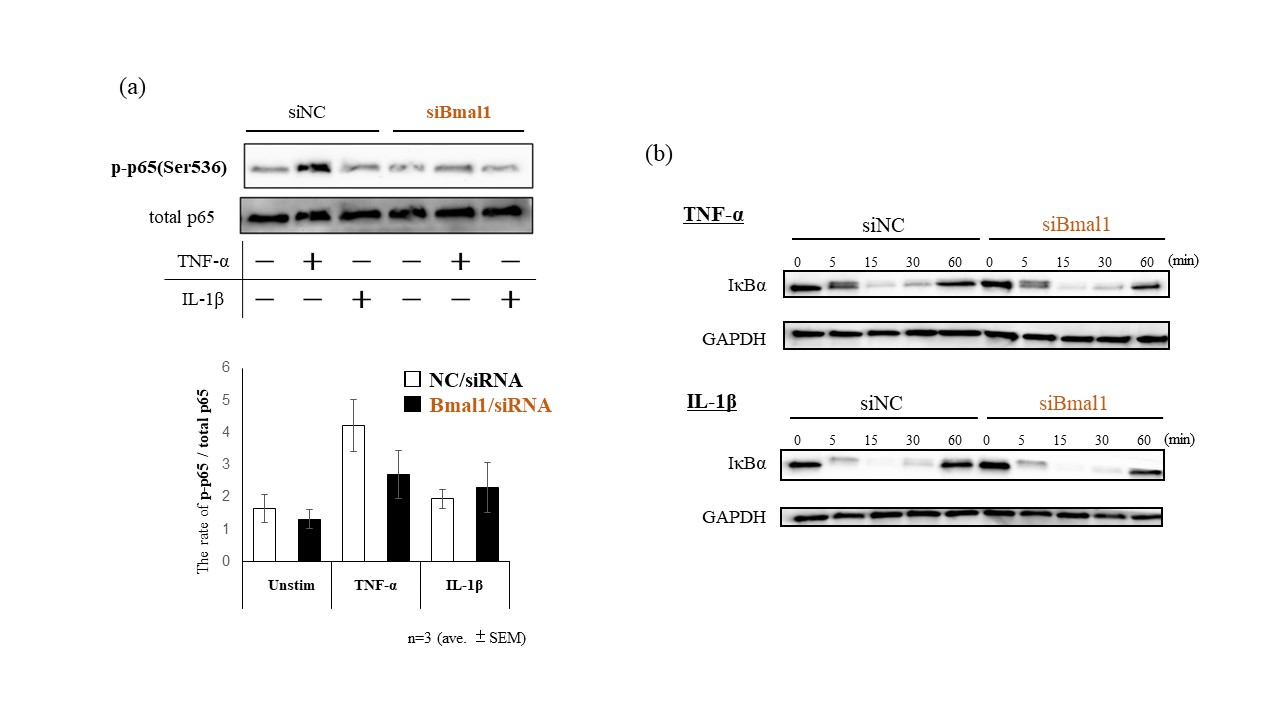
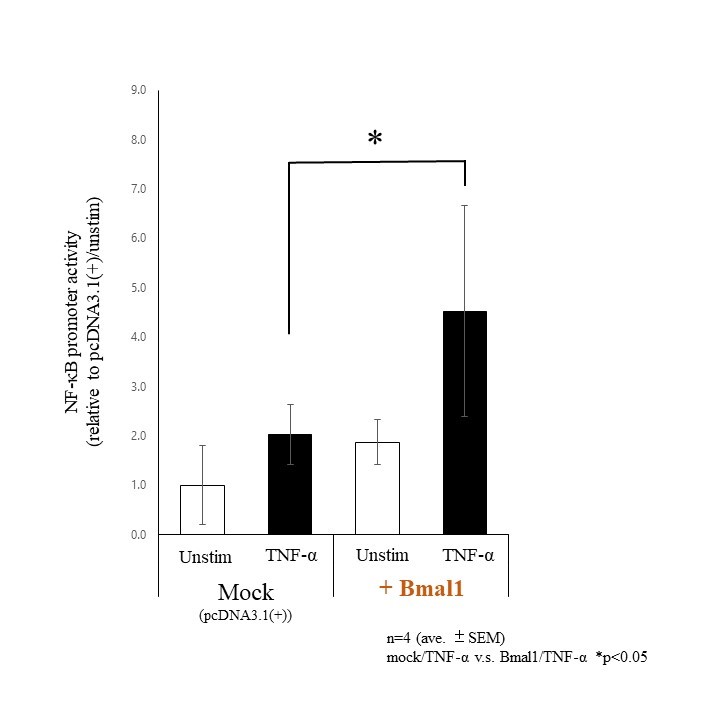
Methods: RA-FLSs were stimulated with or without TNF-α or IL-1β (20 ng/ml) after transfected Bmal1/siRNA, then we investigated either NF-κB/p65 activities by immunofluorescence or the binding of BMAL1 and NF-κB/p65 by co-immunoprecipitation with anti-p65 Abs followed by western blot with anti-BMAL1 Abs. Total protein was extracted to analyze the expression of both IκBα and phospho-/total p65 subunit of NF-κB by western blotting. TNF-α induced transcriptional activities of NF-κB were examined by luciferase assay, under Bmal1 overexpression.
Results: By fluorescence observations, NF-κB/p65 translocated into the nucleus following TNF-α and IL-1β stimulation though it appeared to be suppressed by silencing Bmal1 (Fig 1-a). Co-immunoprecipitation apparently showed the binding of BMAL1 and p65 (Fig 1-b). By suppressing Bmal1, phosphorylation of p65 (Ser536) in RA-FLS was decreased though TNF-α stimulation (Fig 2-a). In contrast, degradation of IκBα was affected by neither TNF-α nor IL-1β stimulation (Fig 2-b). Overexpression of Bmal1 promoted transcriptional activities of NF-κB under TNF-α stimulation (Fig 3).
Conclusion: These results suggest that Bmal1 forms a complex with p65 and promotes the transcriptional activity of NF-κB by regulating phosphorylation at Ser536. This mechanism traced a recently reported non-canonical pathway involving Bmal1 and NF-κB, but not a usual canonical pathway involving IκBα. We propose that Bmal1 increases the transcriptional activity of NF-κB and exacerbate inflammation in RA.
To cite this abstract in AMA style:
Tsukamoto H, Kaneshiro K, Yoshida K, Tateishi K, Terashima Y, Shibanuma N, Sakai Y, Hashiramoto A. Clock Gene Bmal1 Contributes to Inflammation via Phosphorylation of NF-κB/p65 in RA-FLS [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/clock-gene-bmal1-contributes-to-inflammation-via-phosphorylation-of-nf-%ce%bab-p65-in-ra-fls/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clock-gene-bmal1-contributes-to-inflammation-via-phosphorylation-of-nf-%ce%bab-p65-in-ra-fls/