Session Information
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: To describe a single center cohort of rheumatic immune related adverse events (irAEs) observed in patients treated with checkpoint inhibitor therapy (ICI) and to identify potential risk factors for persistent rheumatic disease activity.
Methods: Patients referred to the Cleveland Clinic Department of Rheumatic and Immunologic Diseases for rheumatic irAE were retrospectively included and assessed via chart review (2015-2020). Persistent rheumatic irAE was defined as still requiring treatment for rheumatic irAE at last follow-up.
Results: A total of 112 patients (median age 60 years) were identified. The most common irAE clinical phenotypes included inflammatory arthritis (IA, n=46), sicca syndrome (n=21), and polymyalgia rheumatica (n=19). In the entire cohort ICI therapy was held temporarily for rheumatic irAE in 15 patients (13%) for toxicity and permanently discontinued in 26 (23%). At last follow-up, rheumatic irAE had resolved in 41 patients (37%), whereas 71 patients still required treatment of rheumatic irAE (median follow-up 15 months, range 5-30 months). Among therapies administered for irAE, glucocorticoids (GC) were most commonly prescribed (n=78, 70%), followed by hydroxychloroquine (16%), methotrexate (14%), and anti-IL-6 agents (11%). Patients with persistent rheumatic irAE activity more frequently received GC (p=0.018) and had IA (p=0.02) compared to patients that had resolution of irAE. In multivariate analysis, IA patients were at higher risk of persistent rheumatic irAE (OR 3.5 [1.3-10], p=0.014). The overall mortality rate was 27% (n=30). Risk factors for mortality included tumor progression (OR 15.3 [4-65], p< 0.001) and continued treatment with GC at the last follow-up (OR 5.8 [1.5-29], p=0.016). In a survival analysis, continued GC at the end of follow-up was significantly associated with mortality (HR 4.0 [1.3-12], p=0.0001).
Conclusion: In this single-center retrospective cohort of rheumatic irAEs patients, the presence of ICI-related IA was associated with persistent rheumatic disease activity.
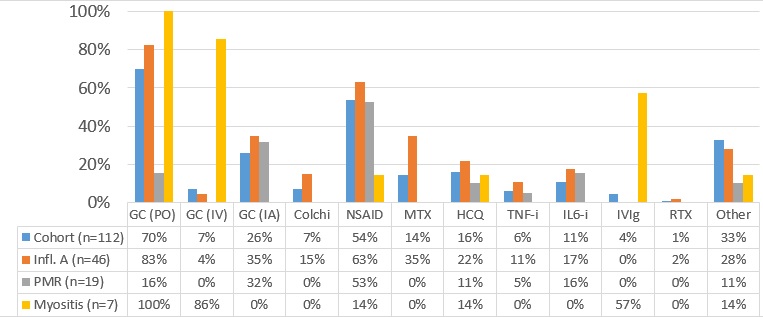 Figure 1. Treatment of rheumatic irAEs Colchi: colchicine; GC: glucocorticoids; HCQ: hydroxychloroquine; Infl. A: inflammatory arthritis; IA: intra-articular; IL6-i: interleukin 6 inhibitors; MTX: methotrexate; RTX: rituximab;
Figure 1. Treatment of rheumatic irAEs Colchi: colchicine; GC: glucocorticoids; HCQ: hydroxychloroquine; Infl. A: inflammatory arthritis; IA: intra-articular; IL6-i: interleukin 6 inhibitors; MTX: methotrexate; RTX: rituximab;
To cite this abstract in AMA style:
Lenfant T, Jin Y, Kirchner E, Funchain P, Song J, Ornstein M, Wood L, Eicher D, Hajj-Ali R, Calabrese L, Calabrese C. Clinical Phenotypes and Treatment of Rheumatic Immune-Related Adverse Events from Checkpoint Inhibitors: A Retrospective Cohort of 112 Patients [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/clinical-phenotypes-and-treatment-of-rheumatic-immune-related-adverse-events-from-checkpoint-inhibitors-a-retrospective-cohort-of-112-patients/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clinical-phenotypes-and-treatment-of-rheumatic-immune-related-adverse-events-from-checkpoint-inhibitors-a-retrospective-cohort-of-112-patients/
