Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Intra-articular corticosteroid injections (IACI) are used to treat active ankle or midfoot inflammation in juvenile idiopathic arthritis (JIA). Ultrasound (US) can help identify the specific joint or periarticular tendon that is inflamed and facilitate precise delivery of the medication. This study compared short-term clinical efficacy between US-guided and non-US-guided injections performed by rheumatologists.
Methods: This retrospective case-control study compared clinical outcomes between US-guided and non-US-guided IACI of the ankle and midfoot joint or tendon sheath in patients with JIA at a single quaternary center. We included patients who received an injection between January 1, 2009 and January 1, 2024, without a systemic medication change 28 days before or after the injection, and with at least 1 clinic follow-up visit. Fisher’s exact test was used to compare proportion of arthritis or tenosynovitis resolution at the immediate post-injection clinic visit (within 6 months). Resolution was defined as absence of swelling, warmth, and/or decreased range of motion thought to be due to active inflammation on a rheumatologist’s documented physical exam. Mann–Whitney U test was applied to analyze the change in physician global assessment (PGA).
Results: 64 US-guided and 77 non-US-guided IACI, from 37 and 54 patients respectively, were included. Polyarticular JIA patients were less likely to have US-guided IACI (Table 1). Non-US-guided IACI were more likely to have been done in patients with a positive cyclic citrullinated peptide (CCP) antibody or positive HLA-B27 antigen. No tendon sheaths were injected in the non-US-guided group (Table 2). In the US-guided group compared to the non-US-guided group, subtalar joints were more likely to be injected, and triamcinolone was more likely to be used than triamcinolone hexacetonide (Table 2). Table 3 demonstrates that arthritis and tenosynovitis clinically resolved at the follow up visit in 78% of US-guided and 67% of non-US-guided IACI, which was not statistically significant (p = 0.19). Evaluation of change in PGA after injection demonstrated that both groups had an average decrease of 20/100 (p = 0.65).
Conclusion: Our study found no significant difference in post-injection remission between US-guided and non-US-guided IACI into the ankle and midfoot from a single center over a 15-year span. The results of this study support our current institutional guidelines which do not explicitly recommend diagnostic and procedural US during IACI for all patients with ankle and midfoot inflammation. A multicenter trial with randomization will be preferred to validate our findings.
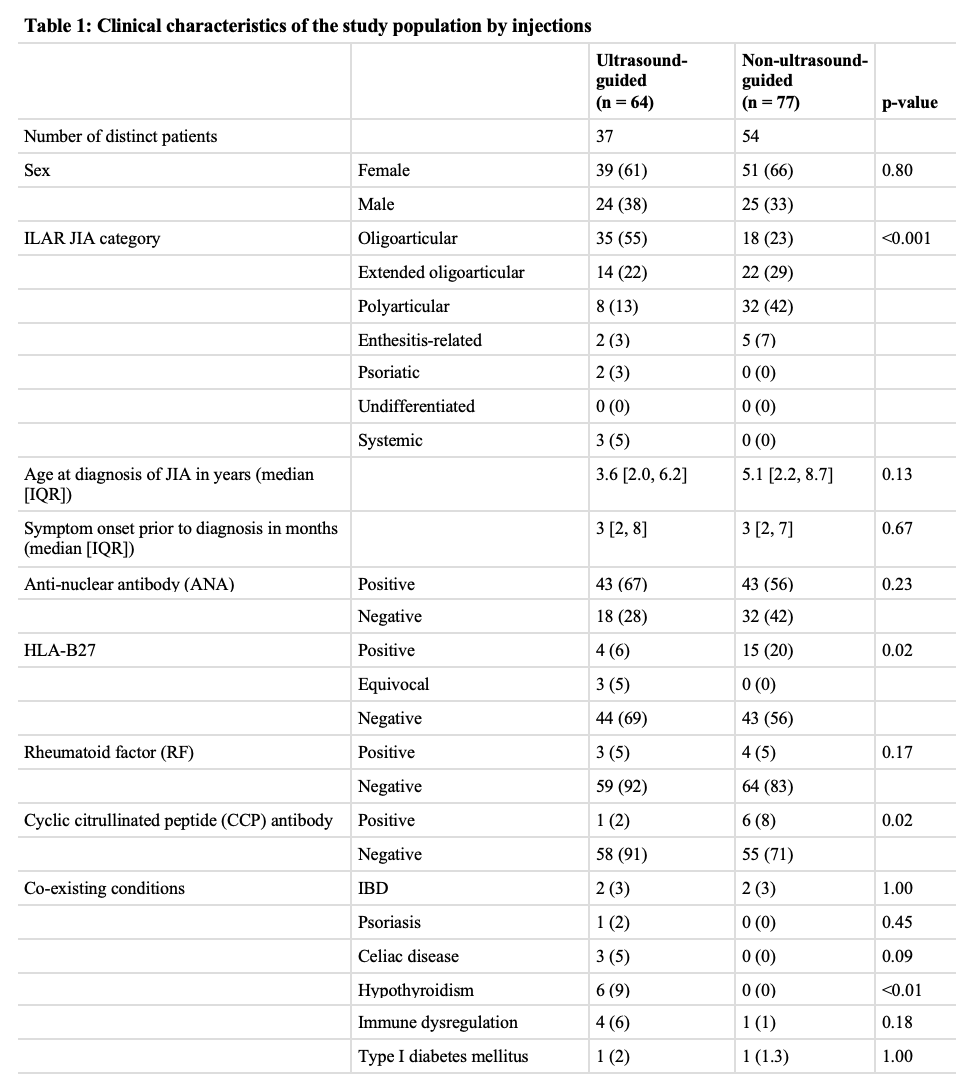 All values are represented as number (%) unless otherwise specified. Some patients had unknown status for ANA, HLA-B27, RF, and/or CCP antibody. ILAR = International League of Associations for Rheumatology, IBD = inflammatory bowel disease (that was diagnosed either before or after JIA was diagnosed); IQR = interquartile range.
All values are represented as number (%) unless otherwise specified. Some patients had unknown status for ANA, HLA-B27, RF, and/or CCP antibody. ILAR = International League of Associations for Rheumatology, IBD = inflammatory bowel disease (that was diagnosed either before or after JIA was diagnosed); IQR = interquartile range.
.jpg) All values are represented as number (%).
All values are represented as number (%).
.jpg) All values are represented as median [IQR] unless otherwise specified. IQR = interquartile range; PGA = physician global assessment (range 0 – 100).
All values are represented as median [IQR] unless otherwise specified. IQR = interquartile range; PGA = physician global assessment (range 0 – 100).
To cite this abstract in AMA style:
Ferguson R, Wang X, Balay-Dustrude E, Iyer R, Rosenwasser N, Shenoi S, Zhao Y. Clinical Outcomes of Ultrasound Guidance for Corticosteroid Injections of the Ankle and Midfoot Joints and Tendon Sheaths in Children with Juvenile Idiopathic Arthritis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/clinical-outcomes-of-ultrasound-guidance-for-corticosteroid-injections-of-the-ankle-and-midfoot-joints-and-tendon-sheaths-in-children-with-juvenile-idiopathic-arthritis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clinical-outcomes-of-ultrasound-guidance-for-corticosteroid-injections-of-the-ankle-and-midfoot-joints-and-tendon-sheaths-in-children-with-juvenile-idiopathic-arthritis/
