Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Tumor necrosis factor inhibitors (TNFi) are effective in children with juvenile spondyloarthritis (JSpA) and generally represent the first-line choice for biologic therapy. However, not all JSpA patients respond well to TNFi initially (primary non-response) or respond well but lose efficacy over time (secondary non-response). Children who do not respond to initial TNF inhibition are left with limited options. We aimed to identify the clinical characteristics associated with TNFi nonresponse in JSpA patients.
Methods: Retrospective analysis of JSpA patients and their response to first TNFi agent enrolled in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry between July 2015 – January 2019. JSpA population included enthesitis-related arthritis, psoriatic arthritis, and undifferentiated spondyloarthritis subtypes. Primary non-response defined as discontinuation of TNFi within the first 3 months of starting. Secondary non-response defined as 1) stopping TNFi AND 2) switching to another biologic or addition of a disease-modifying anti-rheumatic drug (DMARD). Responders were defined those continuing the first TNFi agent for duration of study. Demographic and disease characteristics were compared at 1) baseline visit prior to TNFi start and at 2) time of failure for non-responders or at 4-6 months following TNFi start for responders.
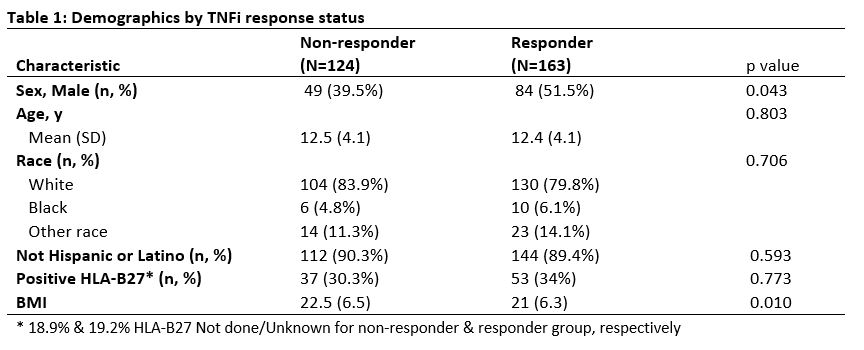
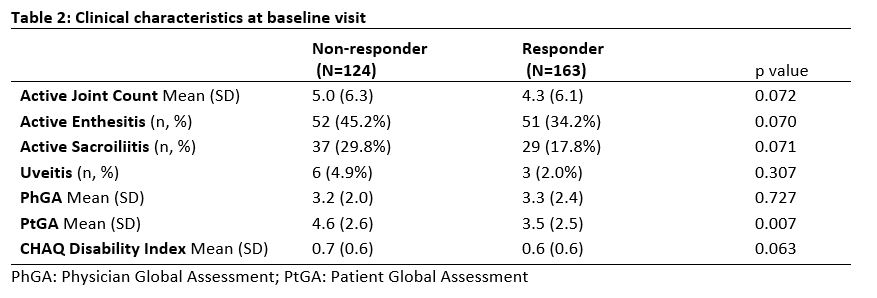
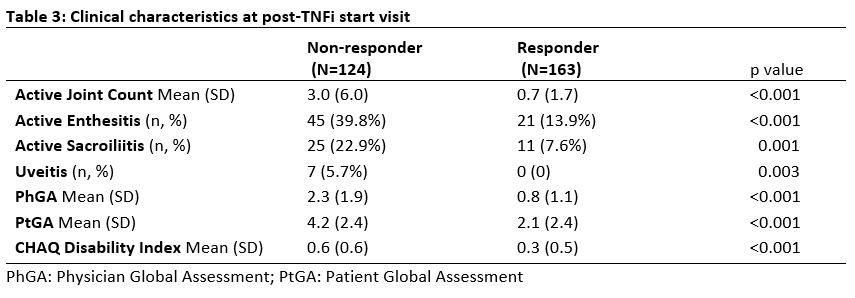
Results: Total of 287 patients met inclusion criteria; 163 TNFi responders and 124 TNFi non-responders. The majority of non-responders were secondary non-responders (91.9%). Median duration prior to initial TNFi failure for non-responders was 274.5 days (IQR:160.5, 433.5). Mean age at TNFi start was 12.5y for non-responders (SD 4.1y) and 12.4y for responders (SD 4.1y) (Table 1). Non-responders were more likely to be female compared to responders, 60.5% vs 48.5% (p=0.04). BMI was higher in non-responders compared to responders, 22.5 vs 21 (p=0.01). Non-responders had more arthritis, enthesitis and sacroiliitis at their baseline visit prior to TNFi start (Table 2) and a post-TNFi start visit (Table 3) compared to responders. Following initial TNFi therapy for all the non-responders, most patients switched to 2nd TNFi (60.2%), followed by DMARD (35.2%) then another biologic (4.6%).
Conclusion: JSpA patients who did not respond to initial TNFi had more active disease and more axial involvement at baseline prior to starting therapy. Additionally, the non-responders were found to have higher BMI compared to the responders.
The authors wish to acknowledge the support from Arthritis Foundation and CARRA. This project is funded by a CARRA-Arthritis Foundation small grant.
To cite this abstract in AMA style:
Oliver M, Mosesso K, Weiss P, Colbert R, Stoll M, Srinivasalu H. Clinical Disease Manifestations Associated with TNF Inhibitor Non-Response in Juvenile Spondyloarthritis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/clinical-disease-manifestations-associated-with-tnf-inhibitor-non-response-in-juvenile-spondyloarthritis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clinical-disease-manifestations-associated-with-tnf-inhibitor-non-response-in-juvenile-spondyloarthritis/