Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Sprifermin is an investigational intra-articular (IA) recombinant human truncated fibroblast growth factor-18 (rhFGF-18) therapy evaluated to date in three knee osteoarthritis (KOA) trials (NCT00911469 [first-in-human {FIH} study]; NCT01033994; NCT01919164 [Phase 2 ‘FORWARD’ study]) as a potential disease modifying OA drug. One of the treatment goals related to slowing OA structural progression is to significantly delay/prevent joint failure. In the FIH study, which enrolled a population planned to undergo knee replacement (KR), and in FORWARD, which enrolled those not planning to undergo KR for at least 2 years, a numeric reduction in KR incidence was seen within the study follow-up period of 6 months or years (Y) 3 to 5, respectively, for the highest doses of sprifermin. This post hoc analysis explores clinical and structural data on FORWARD study participants (pts) who underwent KR in Y3-5.
Methods: Pts with KOA were randomized 1:1:1:1:1 in FORWARD to receive cycles (one IA injection weekly x 3 weeks) of placebo (PBO) or sprifermin at 30 or 100 μg IA every 6 or 12 months (Q6M, Q12M) through month 18, and were followed through to Y2 (treatment period) and Y5 (post-treatment period). Baseline (BL) and week 104 (W104) clinical and imaging data were analyzed post hoc for the population with a BL and ≥1 post-BL MRI (modified intent‑to-treat [mITT]) who completed the treatment period by KR receipt in Y3-5. The Q6M and Q12M data for the sprifermin 30 μg and 100 μg arms were combined for analysis and reported descriptively. No imputation for missing data and no statistical tests were performed.
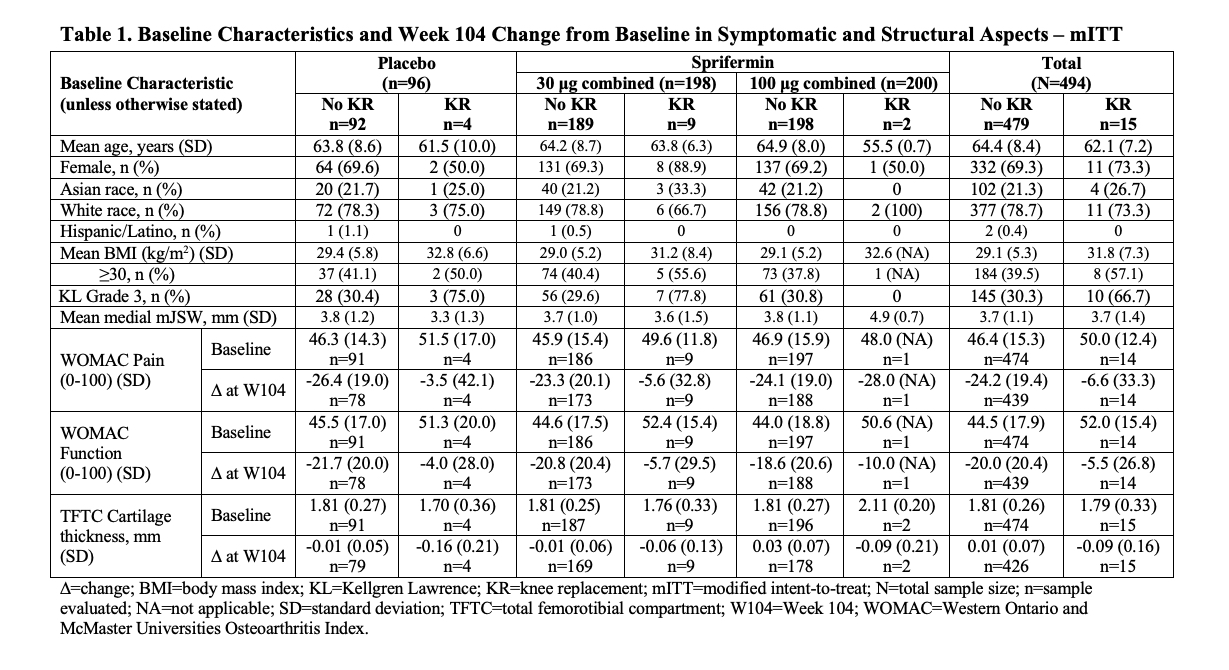
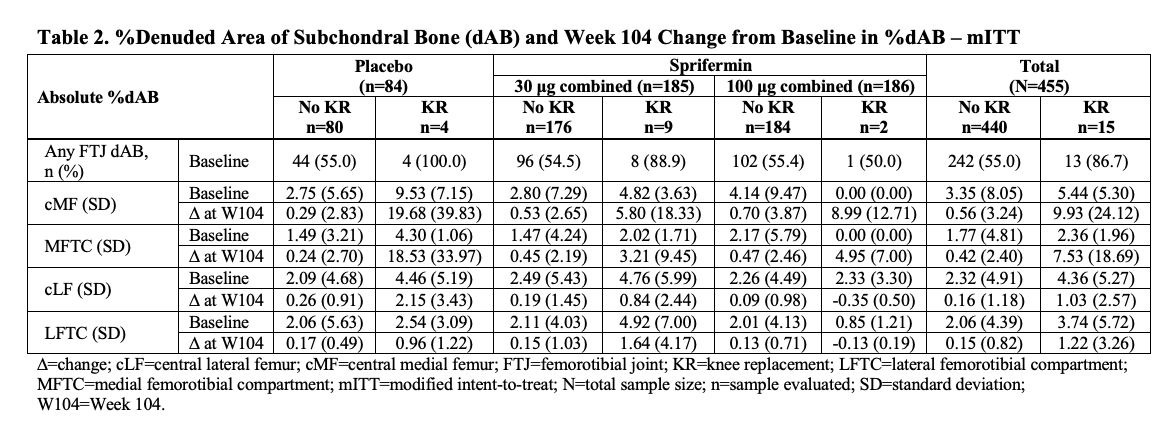
Results: In the PBO and sprifermin 30 μg Q12M, 30 μg Q6M, 100 μg Q12M, and 100 μg Q6M arms, the overall incidence of KR during Y3-5 was 5%, 4%, 5%, 2%, and 0% (n=4/87, 4/99, 5/92, 2/98, and 0/96), respectively. Study pts who received KR were younger than those without (Table 1). Most KRs (67%) occurred in Kellgren-Lawrence grade 3 knees at BL. The total KR population at BL had slightly greater pain, slightly more functional impairment, thinner total femorotibial compartment (TFTC) cartilage, and more denuded cartilage area of subchondral bone (dAB) than the non-KR population (Tables 1 and 2). At W104, the total KR population had numerically less improvement in pain and function, more cartilage thickness loss, and more medial femorotibial compartment (MFTC) dAB than the non-KR population (Tables 1 and 2), more so in the weight-bearing central medial femur [cMF] than in the medial tibia. These changes appeared more pronounced in the PBO group than the sprifermin 30 μg and 100 μg combined groups. Changes in dAB at W104 in the lateral femorotibial compartment (LFTC) were less marked than those in the MFTC.
Conclusion: Pts treated with IA sprifermin 100 μg had a lower incidence of KR than those receiving PBO or sprifermin 30 μg, with the incidence of KR being similar in the latter groups. Although the overall incidence of KR was low, differences in clinical and structural features (especially in the medial compartment) of KOA were apparent at BL and W104, with less symptomatic improvement and more structural worsening at W104, in those who received KR compared to those who did not. These findings need to be confirmed in a prospective study.
To cite this abstract in AMA style:
Conaghan P, Eckstein F, Wirth W, Hochberg M, Guermazi A, Zhao L, Goel N. Clinical and Imaging Characteristics of Individuals Who Underwent Knee Replacement During Years 3 to 5 in the FORWARD Study: A Post Hoc Analysis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/clinical-and-imaging-characteristics-of-individuals-who-underwent-knee-replacement-during-years-3-to-5-in-the-forward-study-a-post-hoc-analysis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clinical-and-imaging-characteristics-of-individuals-who-underwent-knee-replacement-during-years-3-to-5-in-the-forward-study-a-post-hoc-analysis/