Session Information
Date: Sunday, October 26, 2025
Title: (0233–0279) Miscellaneous Rheumatic & Inflammatory Diseases Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Dermatomyositis (DM) is an idiopathic inflammatory myopathy subclassified as classic (CDM) and clinically amyopathic (CADM). It can involve myositis-associated (MAA) and myositis-specific (MSA) autoantibodies. Many MSA are associated with specific DM subsets, which helps in disease monitoring and prognostication. However, few studies have compared the positivity rates of all commonly tested MAA and MSA in CDM versus CADM.
Methods: We performed a retrospective cross-sectional review of CDM and CADM patients in our IRB-approved DM database at the University of Pennsylvania who had myositis autoantibody testing between April 01, 2016 and October 31, 2024. MAA included anti-SSA, -Pm-Scl, -Ku, -U1-RNP, -U2-RNP, and -U3-RNP. MSA included anti-Jo-1, -PL-7, -PL-12, -EJ, -OJ, -Mi-2, -SRP, -TIF-1γ, -NXP-2, -MDA5, and -SAE. ANA status was also recorded. The relation between DM subtype and autoantibody positivity rate was assessed using chi-square and Fisher’s exact tests with α = 0.05.
Results: We identified 320 patients tested at mean age 52.6 ± 14.4 years; 198 (62%) were CDM and 122 (38%) CADM. MSA positivity rate was 47% (94/198) in CDM vs. 40% (49/122) in CADM, p = 0.20. Though typically seen in CADM, anti-MDA5 was comparably positive in CDM (8%, 13/166) and CADM (10%, 10/97), p = 0.49. Although anti-NXP2 was more common in CDM (10%, 16/162) than in CADM (5%, 5/94), the difference was not statistically significant (p = 0.20). Anti-TIF-1γ was the most common MSA in both subtypes (CDM 17%, 29/169; CADM 22%, 21/97). MAA positivity rate was 29% (48/168) in CDM vs. 26% (26/100) in CADM, p = 0.65. Anti-SSA was the most common MAA in both subtypes (CDM 21%, 33/159; CADM 19%, 18/94). ANA was positive in 63% (90/143) CDM vs. 49% (42/85) CADM, p = 0.045. Of the patients with negative myositis panels, 26/53 (49%) CDM and 13/36 (36%) CADM were ANA positive.
Conclusion: We found that MSA and MAA positivity rates did not differ significantly between CDM and CADM (p > 0.05), whereas ANA was significantly more common in CDM (p < 0.05). A sizeable portion of DM was ANA positive despite negative MSA and MAA, which suggests that there may be other DM-related autoantibodies not yet discovered. These results reiterate that DM management requires integrating clinical and lab findings and should not solely depend on autoantibody status.
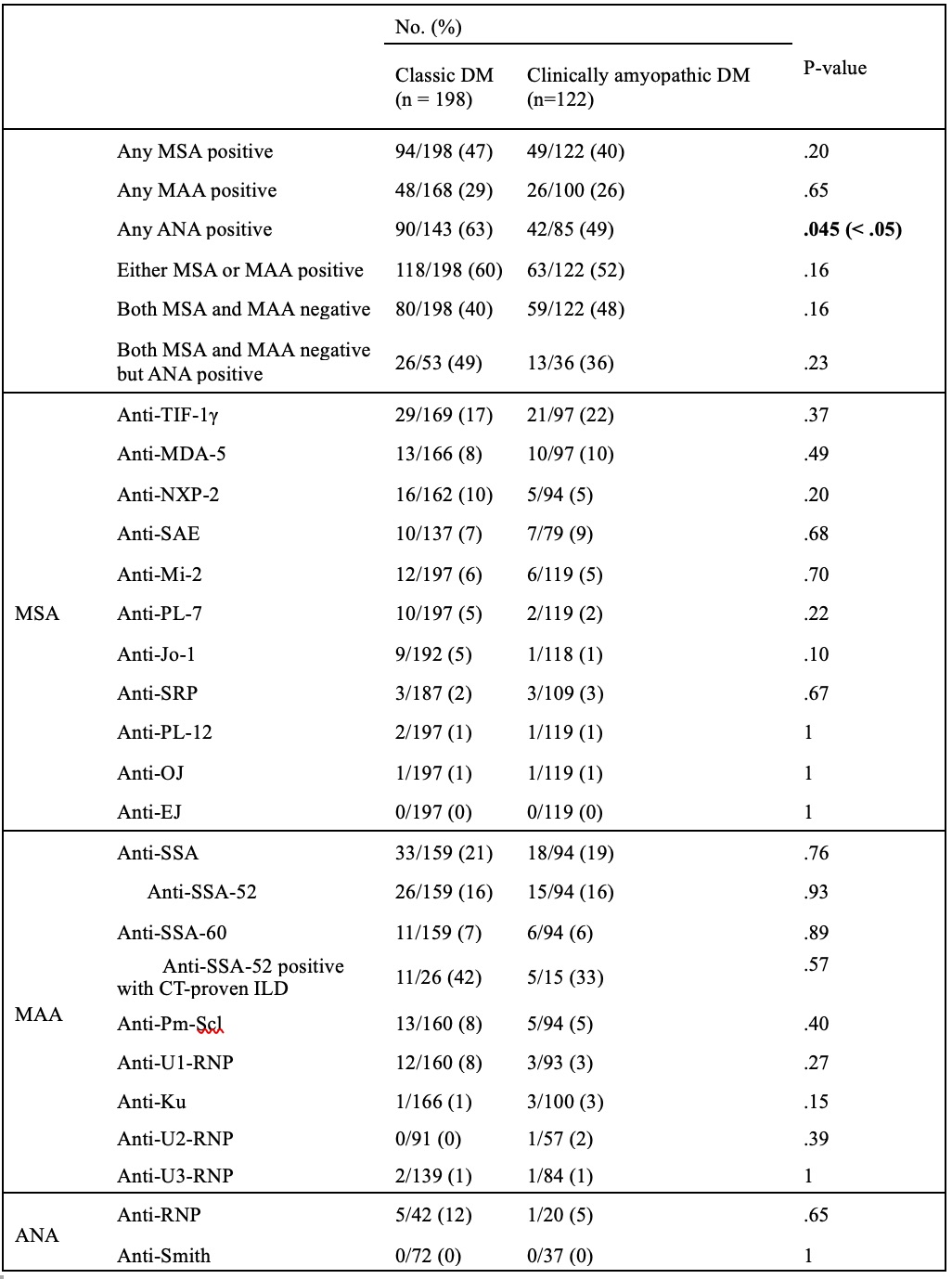 Table 1. Testing Laboratories, Tested Antibodies, and Detection Assays of Myositis Autoantibody Panels Performed in the Study Cohort
Table 1. Testing Laboratories, Tested Antibodies, and Detection Assays of Myositis Autoantibody Panels Performed in the Study Cohort
.jpg) Table 2. Positivity Rates of Myositis-specific (MSA), Myositis-Associated (MAA), and Anti-nuclear Autoantibodies (ANA) by Dermatomyositis (DM) Subtype
Table 2. Positivity Rates of Myositis-specific (MSA), Myositis-Associated (MAA), and Anti-nuclear Autoantibodies (ANA) by Dermatomyositis (DM) Subtype
To cite this abstract in AMA style:
Yang X, Chambers S, On A, Ali H, Khosravi T, Lopes Almeida Gomes L, Feng R, Werth V. Classic and Clinically Amyopathic Dermatomyositis: Autoantibody Positivity [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/classic-and-clinically-amyopathic-dermatomyositis-autoantibody-positivity/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/classic-and-clinically-amyopathic-dermatomyositis-autoantibody-positivity/
