Session Information
Session Type: Abstract Session
Session Time: 4:00PM-5:30PM
Background/Purpose: Systemic Juvenile Idiopathic Arthritis (SJIA) and Macrophage Activation Syndrome (MAS) are associated with highly elevated peripheral blood levels of the inflammasome-activated cytokine IL-18 and chronic exposure to free IL-18. IL-18 canonically acts on NK and activated T-cells in concert with other signals, like T-cell receptor engagement or IL-12 signaling, to amplify Interferon gamma (IFNg) production and cytotoxicity. We sought to determine the mechanisms by which excess IL-18 drives systemic hyperinflammation.
Methods: We assessed lymphocyte phenotypes, distribution, and function via flow cytometry and RNAseq in mice with transgenic expression of mature, secretable IL-18 (Il18tg) and relevant controls. Lymphocytic Choriomeningitis Virus (LCMV) Armstrong strain and ectromelia virus (ECTV) were injected intraperitoneally and via footpad, respectively. Cytokines were measured by bead array and viral quantitation by quantitative PCR. Data were analyzed using descriptive statistics.
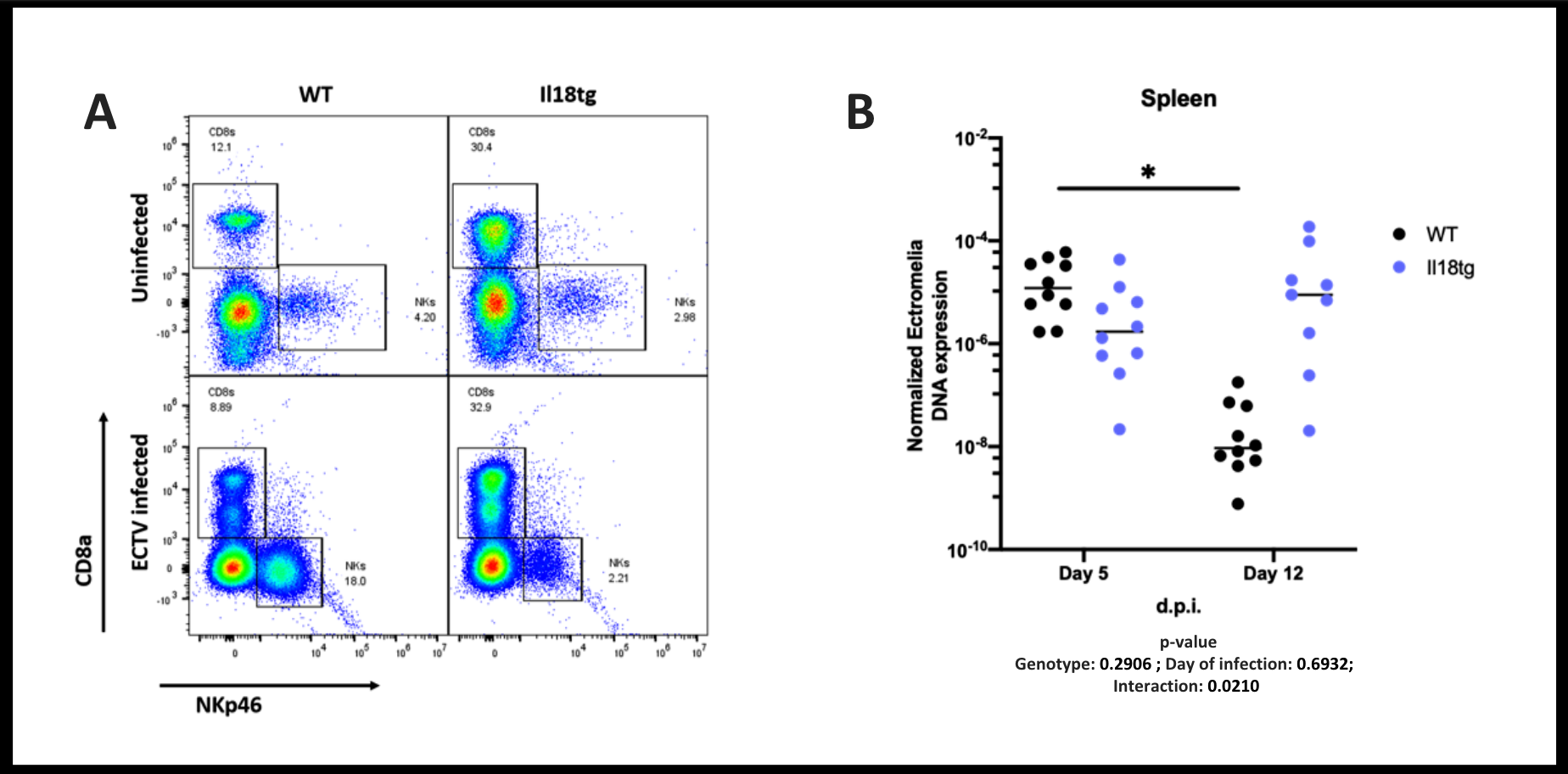
Results: Like SJIA/MAS patients, Il18tg mice demonstrated decreased numbers of NK cells and a concomitant increase in activated CD8 T-cells in peripheral blood, spleen, and liver. Splenic and hepatic NK cells from Il18tg mice showed decreased IL-18 receptor expression, and were enriched for cell cycle and gene transcription programs, suggesting rapid cellular turnover possibly due to activation-induced cell death. Il18tg mice cleared (non-lytic) LCMV infection similarly to WT, but thereafter developed CD8 T-cell and IFNg-mediated immunopathology reminiscent of MAS. To directly challenge NK function in vivo, We infected Il18tg mice with ectromelia, which causes severe viral immunopathology in NK-depleted mice. In contrast to WT, NK cells from infected Il18tg mice neither expand nor upregulated activation markers. However, Il18tg mice managed virus similarly to WT mice at early timepoints. NK depletion impaired viral clearance in WT, but not Il18tg mice. As with LCMV, Il18tg mice developed MAS at 8-12 days post-infection, the peak of the CD8 T-cell response. As expected, WT mice largely cleared ectromelia between days 5 and 12. Il18tg mice showed robust CD8 T-cell expansion and activation, but nevertheless failed to significantly decrease ectromelia DNA (Fig. 1).
Conclusion: Thus, though chronic excess IL-18 both impairs NK cell numbers and IL-18 responsiveness, NK dysfunction appears irrelevant for the development of virus-triggered MAS. Chronic IL-18 excess may overcome NK dysfunction by “pre-priming” CD8 T-cell responses at early timepoints. However, excess IL-18 induced hyperinflammation to a variety of triggers, but only with the lytic ectromelia virus did excess IL-18 also causean unexpected, selective immunodeficiency most evident at the peak of CD8 T-cell expansion. Together, this suggests IL-18 may promote MAS by both CD8 T-cell cytokine amplification and an unexpected impairment of viral clearance.
To cite this abstract in AMA style:
Varghese J, Canna S, Landy E, Eisenlohr L, Peauroi E, Dang V, Frank-Kamenetskii A, Morrissette J. Chronic Excess IL-18 Induces NK Deficiency, but Drives Hyperinflammation via CD8 T-cell Cytokine Overproduction and Selective Immunodeficiency [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/chronic-excess-il-18-induces-nk-deficiency-but-drives-hyperinflammation-via-cd8-t-cell-cytokine-overproduction-and-selective-immunodeficiency/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/chronic-excess-il-18-induces-nk-deficiency-but-drives-hyperinflammation-via-cd8-t-cell-cytokine-overproduction-and-selective-immunodeficiency/