Session Information
Session Type: Abstract Session
Session Time: 1:00PM-2:30PM
Background/Purpose: Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by synovial inflammation due to cellular infiltration and inflammatory mediators. Type I interferons (IFN) have been implicated in the pathogenesis and modulation of autoimmune diseases, including RA, but their role in RA pathogenesis remains poorly understood, partly owing to their complex association with immunity and tolerance. We aim to understand the role of type I IFN signaling in arthritis.
Methods: Zymosan-induced arthritis (ZIA) was induced in IFN alpha receptor 1 null mice (IFNAR1 KO) and C57BL/6J wild-type controls with a 180 µg zymosan intra-articular injection in the right knee using a protocol approved by the Weill Cornell Medicine IACUC. Ipsilateral and contralateral joints were dissected on D7 en bloc for downstream micro-computed tomography (µCT) and histologic analysis to assess synovitis and bone erosion (n=4/group). Slides were H&E-stained and analyzed via automated histomorphometry for synovial area and cell count. The proximal femurs were dissected and the bone marrow was flushed and analyzed for hematopoietic stem and progenitor cells (HSPCs) via flow cytometry (n=4/group). On D7 and D21, peri-articular synovial tissue of the ipsilateral joint was collected for flow cytometry analysis at D7 and D21 (n=5/group). Appropriate t-test and 2-way ANOVA statistical tests were perfomed.
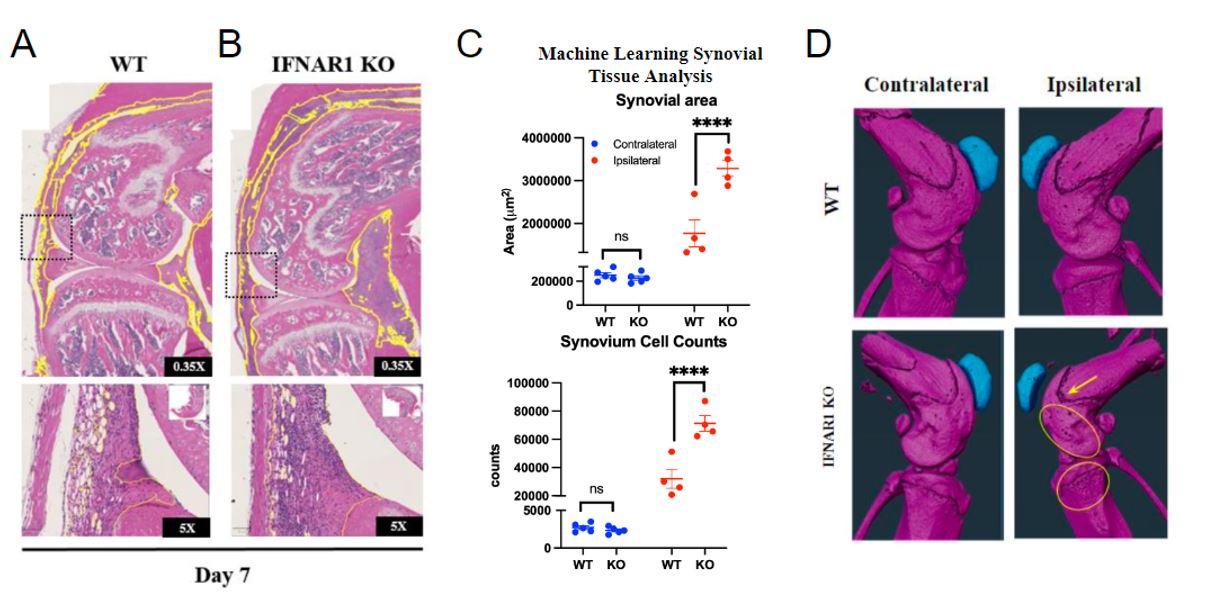
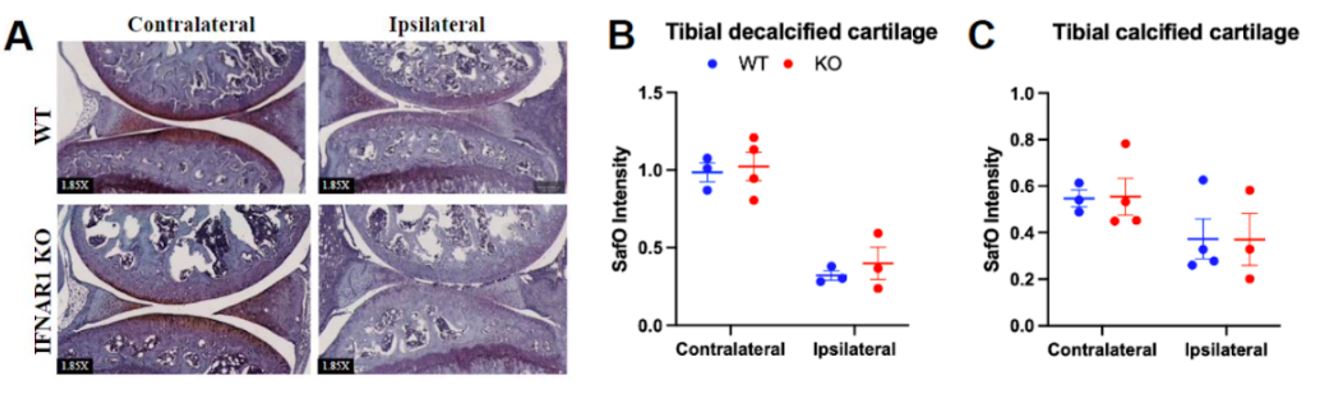
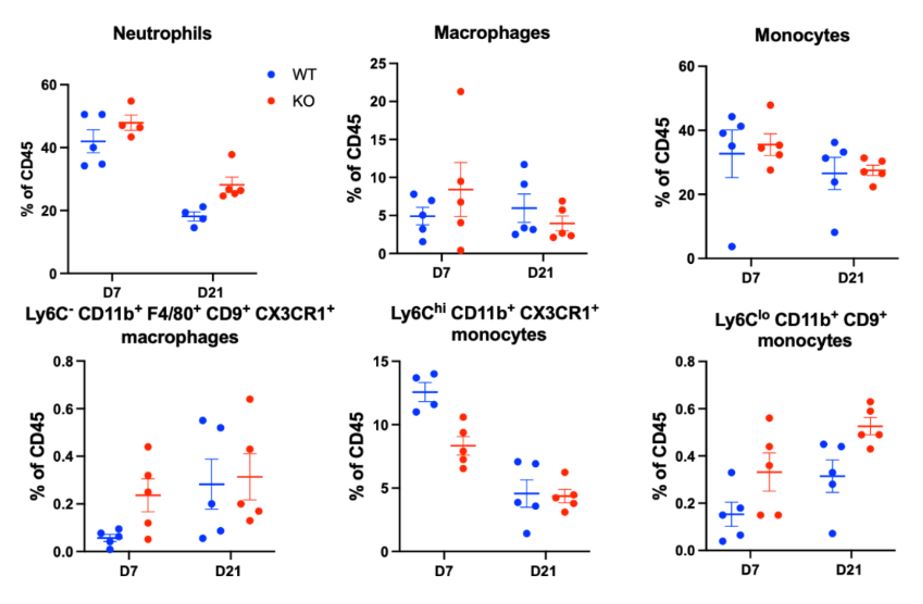
Results: Histologic and µCT analysis of the ipsilateral joint from D7 males revealed significantly increased synovial area (Fig 1 WT: 1.7 x 106 μm2 ± 6.3 x 105 μm2 vs IFNAR KO: 3.2 x 106 μm2 ± 3.7 x 105 μm2, p< 0.01), synovial cell count (WT: 3.2 x 104 cells ± 1.3×104 cells vs KO: 7.1 x 104 cells ± 1.1 x 104 cells, p< 0.01) and bone erosion in IFNAR1 KO mice compared to WT on D7 post zymosan injection (Fig 1). We did not observe changes in levels of Lin-Sca1+cKit+ HSPCs, an early progenitor cell, multipotent progenitors (MPPs), short-term hematopoietic stem cells (ST-HSCs), long-term HSCs (LT-HSCs), or MPP2, MPP3, and MPP4 cells in KO with ZIA (not shown). Tibial cartilage proteoglycan content did not differ between KO and WT (Fig 2). Flow cytometry analysis of synovial tissue from the ipsilateral knee shows significantly increased neutrophils at D21 (p=0.05) and significantly decreased Ly6ChiCD11b+CX3CR1+ monocytes at D7 (p=0.01) (Fig 3).
Conclusion: Our data demonstrate that ZIA in IFNAR1 KO increases synovitis at D7, but does not affect myelopoiesis or cartilage degradation at day 7 post zymosan injection. Unexpectedly, levels of LSK cells and MPPs were unchanged at D7 in IFNAR1 KO despite increased synovitis. However, defining potential epigenetic and transcriptomic changes in bone marrow HSPCs and myeloid progenitors in IFNAR1 KO subjected to ZIA remains an area of future investigation. The observed increased inflammatory neutrophils yet decreased Ly6ChiCD11b+CX3CR1+ monocytes, which may be an inflammatory monocyte population, recapitulates the functionally heterogeneous outcomes known to occur downstream of IFNAR1. Further characterization of IFNAR1 KO may help resolve the cell-type specific activities catalyzed by IFNAR1 and has the potential to enable future type I interferon-specific therapeutics for inflammatory disease.
To cite this abstract in AMA style:
Huang M, Bell R, Singh T, Ivashkiv L. Characterizing the Functional Role of Type I Interferons in Inflammatory Arthritis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/characterizing-the-functional-role-of-type-i-interferons-in-inflammatory-arthritis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/characterizing-the-functional-role-of-type-i-interferons-in-inflammatory-arthritis/