Session Information
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: Many osteoarthritis (OA) patients develop fibrosis of the synovial membrane leading to joint swelling, stiffness, and pain. Synovial fibroblasts activated in the synovial membrane are responsible for excessive extracellular matrix (ECM) formation and are key mediators of synovial fibrosis. There are currently no disease-modifying treatments approved for OA. A better understanding of the activating mediators of fibrosis and the deposited matrix tissue profile as a consequence may help guide the development of potential new treatments in OA. Nintedanib and Tofacitinib are treatments approved for anti-fibrotic treatment of fibrotic conditions and anti-inflammatory treatment of autoimmune disease respectively. In this study, we aimed to characterize the ECM profile of OA fibroblast-like synoviocytes (FLS) in response to the known joint-related growth factor TGF-β1 and assess the effect of known anti-fibrotic and anti-inflammatory treatments on biomarkers of fibrogenesis.
Methods: Primary human FLS were isolated from synovial membranes extracted from 10 OA patients undergoing total knee replacement. Fibroblasts were grown in DMEM media containing 0.4% fetal calf serum, ficoll (to produce a crowded environment), and ascorbic acid for 12 days. Cells were cultured either without treatment (untreated), with TGF-β1 [2 ng/ml] or TGF-β1 [2 ng/ml] together with Nintedanib [1 µM, 0.1 µM, 0.01 µM] or Tofacitinib [100 µM, 50 µM, 25 µM], and treatment changed at day 4 and 8. Type I, III, and VI collagen formation reflecting tissue fibrogenesis (PRO-C1, PRO-C3, and PRO-C6, respectively) were evaluated by validated ELISAs in the supernatant from days 0, 4, 8, and 12 and metabolic activity was measured as a surrogate of viability using Alamar Blue on day 0 and 12.
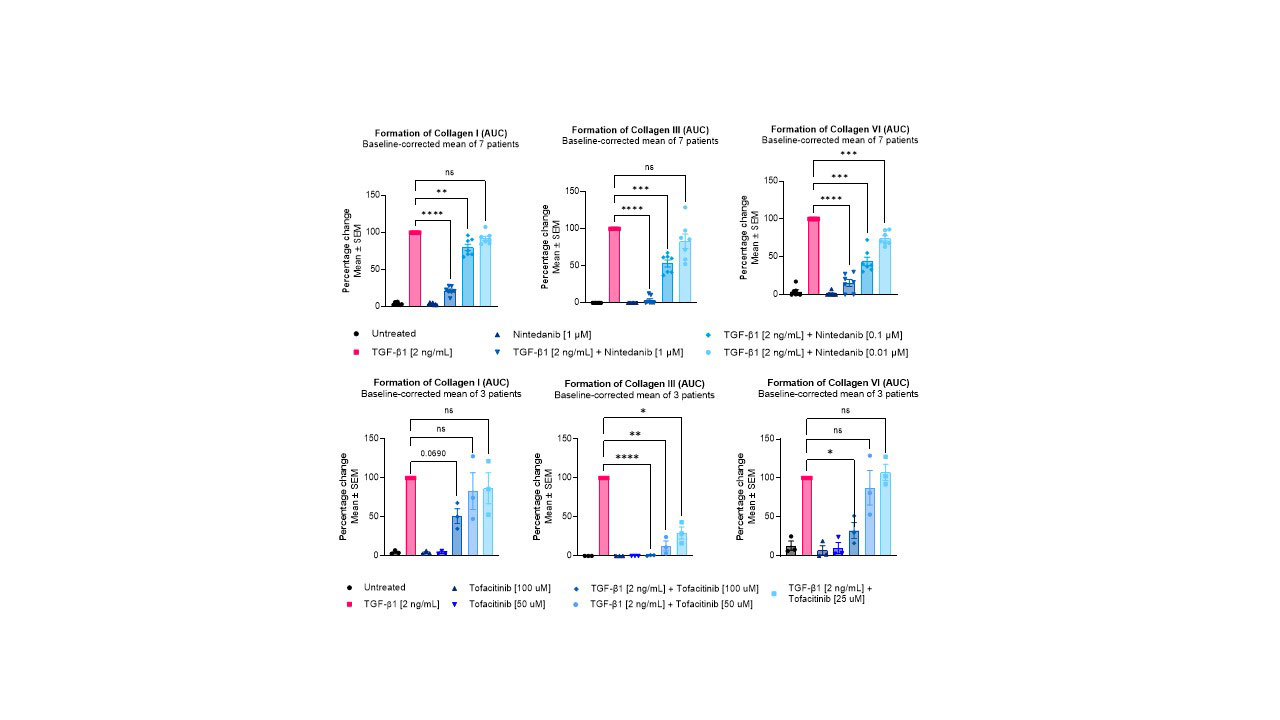
Results: TGF-β1 stimulation increased type I, III, and VI collagen formation from day 4 through to day 12, compared to untreated cells. Treatment with either Nintedanib or Tofacitinib reduced the metabolic activity to pre-TGF-β1 stimulated levels. Nintedanib reduced all three markers of collagen formation in a dose-dependent manner at both 1 µM (PRO-C1: 78%; PRO-C3: 96%; PRO-C6: 84%) and 0.1 µM (PRO-C1: 20%; PRO-C3: 47%; PRO-C6: 56%), and at 0.01 µM for PRO-C6 (25%), compared to TGF-β1 alone (figure 1). Treatment with Tofacitinib reduced the PRO-C1 and PRO-C6 levels by 49% and 67 %, respectively, at 100 µM, while no significant effect was observed at the lower doses. Furthermore, Tofacitinib inhibited PRO-C3 dose-dependently by 99%, 89%, and 84% at doses 100, 50, and 25 µM, respectively, compared to TGF-β1 control.
Conclusion: In summary, TGF-β1 induces a fibrogenic response in fibroblast-like synoviocytes, which is characterized by increases in type I, III, and VI collagen. This fibrotic response is reduced by the anti-fibrotic drug Nintedanib, as well as the anti-inflammatory JAK inhibitor Tofacitinib. These data suggest that JAK inhibitors may possess anti-fibrotic properties and could be explored as anti-fibrotic treatments in synovial fibrosis.
To cite this abstract in AMA style:
Falkenløve Madsen S, Madsen S, Hollaar E, Gantzel T, Bay-Jensen A, Thudium C. Characterizing the Anti-fibrotic Effect of Tofacitinib in TGF-β Stimulated Fibroblast-like Synoviocytes from Patients with OA [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/characterizing-the-anti-fibrotic-effect-of-tofacitinib-in-tgf-%ce%b2-stimulated-fibroblast-like-synoviocytes-from-patients-with-oa/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/characterizing-the-anti-fibrotic-effect-of-tofacitinib-in-tgf-%ce%b2-stimulated-fibroblast-like-synoviocytes-from-patients-with-oa/